Application of WDXRF and FT-IR for Human Tooth Analysis
Spectroscopy
In this study, WDXRF and FT-IR are used to analyze a tooth sample of a renal patient, and to compare the results to healthy patients. The quantities of multiple elements are reported using the XRF technique, and FT-IR spectroscopy is used to extract relevant information about the molecular contents of the sample with the important absorption bands identified.
In this study, we analyzed a tooth sample of a renal patient using wavelength-dispersive X-ray fluorescence (WDXRF) spectrometry and Fourier transform infrared (FT-IR) spectrometry to quantify major and trace elements, and heavy and toxic elements in particular. The elements detected and quantified in the tooth sample were calcium (Ca), phosphorus (P), carbon (C), oxygen (O), magnesium (Mg), sodium (Na), chlorine (Cl), sulfur (S), potassium (K), silicon (Si), iron (Fe), aluminum (Al), zinc (Zn), strontium (Sr), chromium (Cr), nickel (Ni), copper (Cu), and zirconium (Zr). FT-IR spectrometry has been used to extract relevant information about the molecular contents of the tooth sample. We have compared our results with those obtained in the laboratory for the healthy subjects, and also with the values reported in the literature as obtained using XRF spectrometry only. Elements found in the tooth sample were correlated with the chronic kidney disease (CKD) of the patients. An outline of the XRF apparatus and applications of XRF as a tool for analyzing to dental specimens are also briefly highlighted.
Vivek K. Singh, Shikha Sharma, Mahima Sharma, Neha Sharma, Jitendra Sharma, and Pradeep K. Rai
Calcified tissues, such as bones and teeth, contain biological signatures from their living phase. The habitat and nutritional and environmental conditions of people can be determined by monitoring the trace elements present in these samples (1). This is not unexpected, as teeth have both an organic and inorganic phase. Teeth and bones consist of an inorganic calcium phosphate mineral approximated by hydroxylapatite and matrix proteins with a small amount of proteolglycans and lipids (2). In contrast, the inorganic phase is composed of well-packed nanocrystals of calcium apatite, together with trace elements (3). The trace elements in the tooth can vary, depending on the source of food and water, as well as from soil and dermal absorption (1–4). Accurate determination of trace elements in calcified tissues still remains a challenge for biomedical researchers. X-ray fluorescence (XRF) spectrometry offers a reliable approach to provide rapid multi-elemental analysis of biological samples in µg/g (ppm) range, and it is a non-destructive technique that allows samples to be preserved for further experimentation (5). Instrumentation, such as wavelength-dispersive XRF (WDXRF), can now be effectively used to accurately measure trace elements in the human tooth. Such information not only provides a useful assessment of the diet, but reflects specific environmental and disease conditions as well.
Recent biochemical studies provide insight into the factors controlling the formation and growth of bioapatite crystals, and how alterations in the mineralization process can lead to diseases such as osteoporosis. The relationship between chronic kidney disease (CKD), specifically hypercalciuric nephrolithiasis, osteoporosis, and bone mineral density (BMD), is well established. A large number of epidemiologic studies linked to evaluating factors associated with BMD in men have amply demonstrated that history of renal disease is indeed associated with lower BMD (6). The kidneys play an important role in maintaining healthy bone mass and structure by balancing calcium (Ca) and phosphorus (P) levels in the blood. Mineral and bone disorders in CKD occur when damaged kidneys and abnormal hormone levels cause Ca and P levels in a patient’s blood to be out of balance. CKD causes mineral and bone disorders, and thus kidneys stop activating calcitriol. The low levels of calcitriol in the body in turn create an imbalance of Ca in the blood by not removing the P in the blood proportionately, so the P level rises in the blood. The extra P pulls Ca out of the bones, causing them to weaken. Consequently, bone losses from the jaw occur in the CKD patients. Such weaker bones can cause teeth to become loose, and eventually fall out.
Therefore, in this paper, we present results from our investigation of major (Ca, P, magnesium [Mg], sodium [Na], chlorine [Cl], sulfur [S], potassium [K], silicon [Si], iron [Fe], aluminum [Al], zinc [Zn], strontium [Sr}, chromium [Cr], nickel [Ni], copper [Cu], and zirconium [Zr]), trace elements, and toxic metals using WDXRF spectrometry in a tooth sample obtained from a CKD patient. We have compared our results with literature reported values (using XRF spectrometry only) and laboratory values for healthy subjects. Fourier transform infrared (FT-IR) spectrometry has also been used to investigate the phosphate, carbonate, and amide contents of teeth samples. This paper presents a very crucial application of WDXRF spectrometry to quantify heavy and toxic elements of calcified tissue materials like tooth samples from renal patients with CKD.
Materials and Methods
Tooth Sample
The tooth sample from the CKD patient was kindly provided by the Department of Nephrology at Opal Hospital in Varanasi, India. In the present work, the studied tooth sample was one that had become loose, and had eventually fallen out of the jaw of a CKD patient. The received tooth sample was dried and stored in a sealed pot after washing in deionized water to remove all traces of possible contaminants. The as-received tooth sample was examined visually, to check for caries. The tooth sample was not affected by caries, and therefore the present study was not influenced by caries formation. The tooth was then powdered using a mortar and pestle, and the powdered tooth sample was used subsequently for experimental measurements using FT-IR and WDXRF spectrometry.
FT-IR Spectrometry
The ground tooth sample was used to record the FT-IR spectrum in a transmittance mode setting for the spectral region of 4000-400 cm−1, with a spectral resolution of 4 cm−1 on an IRTracer-100 Fourier transform infrared spectrophotometer (Shimadzu), using a single-reflection attenuated total reflection (ATR) accessory. In this spectrophotometer, the FT-IR spectrum is recorded in a very simple manner, as only few milligram (mg) of sample (solid or liquid ) are required to place on the surface of the ATR prism. However, solid samples must be held on the surface of the prism using the provided pressure clamp. The spectra can be recorded either in absorption mode or in transmission mode.
WDXRF Spectrometry
XRF analysis provides elemental mapping and imaging information using emitted characteristic X-rays from the sample. Figure 1 shows the mechanism of characteristic X-ray generation. With the bombardment of high-energy electrons or high-energy X-rays, a bound electron is struck out of the target atom. After the electron is ejected, an outer shell electron falls into the vacant inner shell, resulting in the emission of characteristic X-rays with an energy equal to the difference of energy between the outer and inner shell energy levels. These X-rays generated through the bombardment of high energy X-ray irradiation are termed fluorescent X-rays. Each element with inherently different energy levels (for the set of electrons it possesses) emits X-rays of energies that uniquely identify this characteristic element. Therefore, such characteristic X-rays can easily be exploited to perform elemental analysis of a sample. Further details of the theory and applications of XRF spectrometry can be found in reference sources (7–11).
Figure 1: The mechanism of X-ray generation.
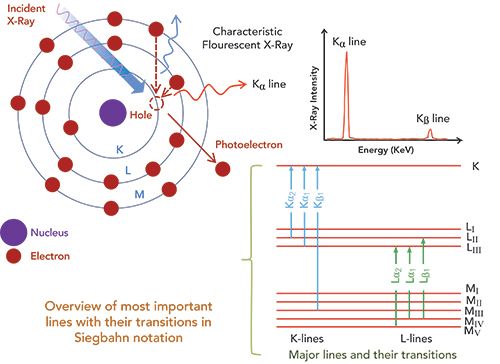
High performance WDXRF spectrometry is now being used for the determination of traces in plant materials, biological samples, and geological samples. To fulfill today’s requirements for quality data, modern instruments must provide high elemental sensitivities to achieve low detection limits and optimal spectral resolution to minimize line overlays. In this work, we have used a WDXRF spectrometer S8 Tiger (Bruker) for the quantitative measurements of major, trace, and heavy elements in the sample. It provided the optimum quantitative results in terms of detection limits, resolution, and reliability for each element with the best excitation and highest intensity over the entire elemental range.
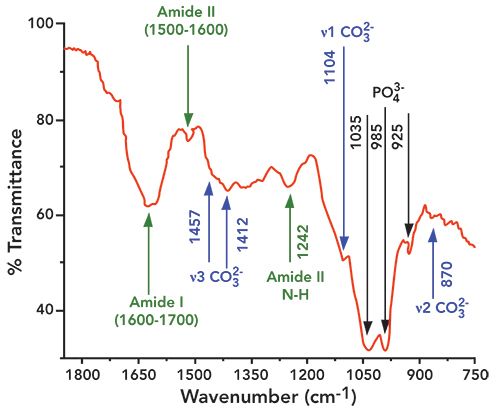
Figure 2: Fourier transform infrared (FT-IR) spectra of the tooth sample in the spectral region of 1800 to 750 cm-1, with the contributions ofand amide I, II, and III shown.
In this WDXRF spectrometer, an end-window X-ray tube with 45Rh anode is adjusted to a voltage of 60 kV and a current of 170 mA, correlating to a maximum power supply of 4kW to generate the primary beam. To ensure more accurate and reproducible results, the tooth sample was analyzed three times. For the detection of lighter elements, a flow proportional counter is used, and, subsequently, a scintillation counter detector is used to detect heavier elements. The acquisition time taken to capture a single spectrum of the sample was 20−25 min. The analyzer crystal LiF(220) ensures that in combination with the 0.23° collimator, the best separation is achieved for adjacent elements. The mask size was 23 mm, and the spectrum was recorded in vacuum. Technical details of the spectrometer used in the present study can be found elsewhere (12). In the present work, while analyzing data, the maximum relative standard deviation (RSD) obtained was 5%. To measure the concentration of trace and heavy elements, the S8 Tiger WDXRF spectrometer uses a standardless semi-quantitative Spectraplus software (Quant Express) package. The technical details of this software can be found elsewhere (12). The unique feature of this software is that it corrects all the matrix effects based on theoretically fundamental physical parameters, such as absorption coefficients and secondary fluorescence enhancement, automatically. In this software, the elemental concentration has been determined using Sherman’s equation (13):
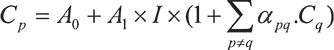
where Cp is the element concentration and I is the measured intensity of the corresponding line; Cq are the concentrations of the other elements q; and A0 and A1 are the coefficients of the calibration regression line, respectively, namely offset and slope. The advantage of this software is that it does not need any standard reference materials to check its reliability and reproducibility with respect to the given samples.
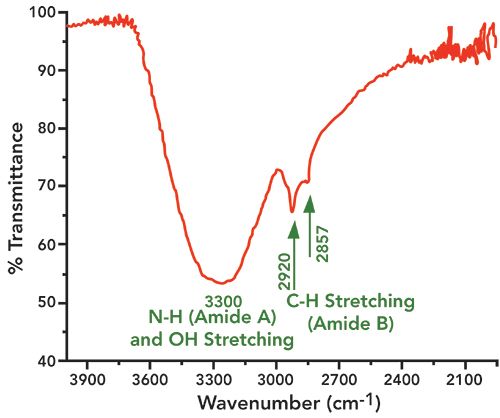
Figure 3: Fourier transform infrared spectra of tooth sample in the spectral region of 4000 to 2100 cm-1, with the contributions of amide A and B and water (-OH stretching) shown.
Because of the limited amount of the tooth sample, we have used the sandwich method to create a pellet. The powdered tooth samples are pressed onto approximately two spatulas full of boric acid using a constant pressure of 15 tons for 15 s on the stainless steel die head using a hydraulic press to prepare a smooth and uniform surface. This method is generally used in cases of limited sample size, and, in such cases, the use of a non backscattering boric acid matrix is recommended (7–11).
Results and Discussion
Analysis of Tooth Sample Using FT-IR Spectrometry
Unless otherwise noted, the literature citations for band assignments are given in references (14,15). The FT-IR spectra of the tooth sample are shown in Figures 2 and 3, and the functional groups found in the sample are indicated in their respective wide scan FT-IR spectra (Figures 2 and 3). A typical -OH stretching band is clearly visible at 3300 cm-1. The characteristics peaks of phosphates appear at 579, 590, 608, and 614 cm−1 (ν4 asymmetric bending mode of ), 1023, 1043, 1052, and 1069 cm−1 (ν3 asymmetric stretching mode of ), 431, and 446 cm−1 (ν2 symmetric bending mode of ), and 960 cm−1 (ν2 symmetric stretching mode of ). Phosphates in the sample are identified by the characteristic absorption band at 1035 cm-1, which appears due to the ν3 asymmetric stretching of . Other phosphate bands (relatively broader) appeared around at 985 and 925 cm-1. The major component of ν2 stretching in , is assigned to type B carbonate
( ions substituting sites), located at 870 cm-1. The type B carbonate ν3 mode presented two bands, one at 1412 cm-1, and the other at 1457 cm-1, respectively, while type B carbonate ν1 mode presented a band around 1069 cm-1 (shoulder peak) (8). Type A carbonate (substituting sites) in the ν3 domain was located at 1545 cm-1, while the Type A carbonate in the ν1 domain was found at 1104 cm-1.
The four primary amide bands are amide I (1700−1600 cm−1), amide II (1600−1500 cm−1), amide A (3400−3350 cm−1), and amide B (3085−3070 cm−1). The absorption peak near 1242 cm-1 represents amide III
(N–H stretching vibration). The amide I band is representative of the secondary structure of proteins. It can be decomposed into three main components; the most intense being the central one, at about 1660 cm−1, which has low- and high-frequency shoulders. Usually a shoulder peak is observed near 1620 cm−1, which is attributed to the amide I feature of dentin and hydrated gel-like collagen. In the present study, the amide I vibrations arising from C=O stretching were centered in the broader 1627 cm-1 band, with shoulders at 1604 and 1665 cm−1, respectively. The amide A region, where the N–H stretching vibrations of the amide normally appear, had its major intensity peak near 3300 cm-1 (overlapped with an OH stretching broadband around 3300 cm-1). The amide B region, arising from C–H stretching, exhibited a band with higher intensities at 2857 and 2927 cm-1, respectively. Absorption peaks centered at 1242 cm-1 have been characterized as amide III (N–H stretching vibrations).
Analysis of Tooth Sample Using WDXRF Spectrometry
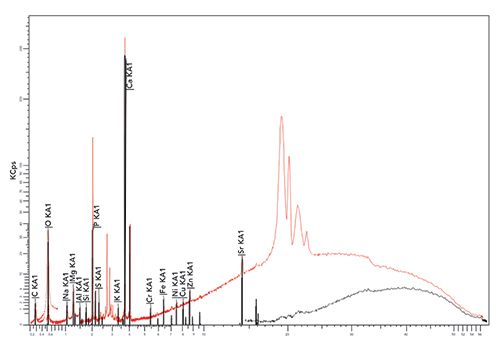
The concentration of different elements present in the tooth sample was determined using WDXRF spectrometry measurements, and the values obtained are presented in Table I. The major and trace elements detected and quantified in the sample were calcium (Ca), phosphorus (P), carbon (C), oxygen (O), magnesium (Mg), sodium (Na), chlorine (Cl), sulfur (S), potassium (K), silicon (Si), iron (Fe), aluminum (Al), zinc (Zn), strontium (Sr), chromium (Cr), nickel (Ni), copper (Cu), and zirconium (Zr). Figure 4 depicts a typical WDXRF spectrum of the tooth sample obtained from the WDXRF spectrometer indicating the different elements present in it. In Table I, we have also summarized the concentration of major and trace elements found in the sample and compared them with those reported in the literature using only XRF techniques (16–21). Similarly, Table II presents the concentration of some major elements, including Ca, P, Mg, Na, K, and Cl, found in the studied sample, along with the average values for sound human enamel and dentin as reported by different laboratories (22–26). Here, to compare with our data, the concentration values from the healthy teeth were considered in the same way as we have analyzed the tooth sample from CKD patients. For the present study, we have analyzed the whole tooth, including enamel and dentine, by making them into a powder. As a constraint (refer to Table II), we are bound to compare our results with the literature-reported values of healthy subjects only, and not with the healthy teeth of the same CKD patient because we are unable to collect healthy teeth from the same patient.
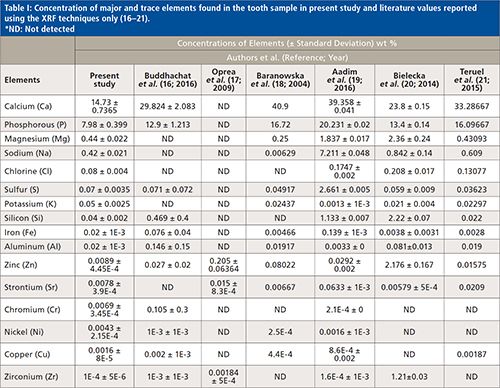
Figure 4: A typical WDXRF spectra of tooth sample obtained from WDXRF spectrometer. The broader peaks in the red spectra indicate the
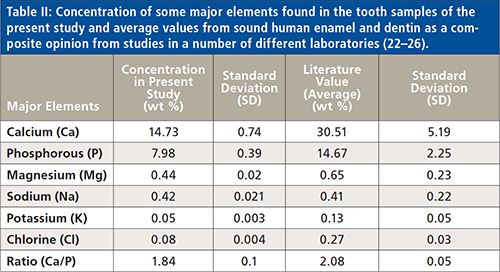
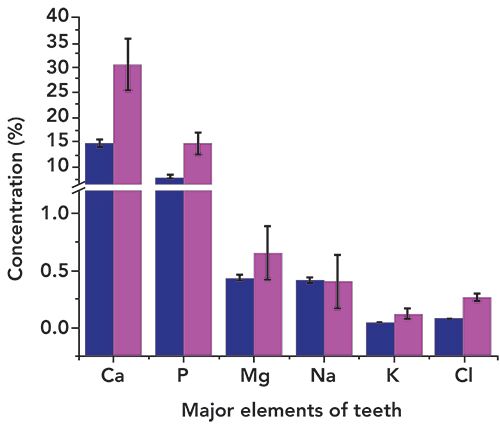
Organic matrix elements detected in the tooth sample were carbon (C) and oxygen (O). The elements Ca (14.73%), and P (7.98%) were the most abundant elements in the studied sample, as substantiated by other reports as well (16–26). It is clear from Table II that the concentrations of elements Ca, P, Mg, K, and Cl are lower in the tooth sample of the CKD patient than that from healthy teeth. Figure 5 clearly shows the relative concentration of such elements in the studied tooth sample. No such variation was observed for Na. The Ca to P ratio was also not consistent with the literature reported value. We noted that CKD apparently caused the imbalance of P in the blood, which in turn affects the Ca in tooth and bone, which is considered as being a major cause of imbalance of Ca in tooth and bone. Reduced amounts of Ca, P, and other major elements (necessary for healthy teeth) were indicated in the tooth sample and may have been caused by CKD and its consequences on the patient.
Figure 5: Comparison of the concentrations of some major elements (Ca, P, Mg, Na, K, and Cl) comparing the present study (dark blue) to the literature values (fuchsia) (References 22–26).
Most of the elements detected in the tooth were those that accumulated within the structure of hydroxyapatite over time. Numerous substitution metals can be found in hydroxyapatite crystals; metal cations such as K+, Na+, Ni2+, Cu2+, Mg2+, or Zn2+ can replace Ca2+, whereas anionic complexes, including , or , can provide a substitute for , and anions such as Cl- or F- can occupy the sites of OH- in the crystal structure (26–29). Therefore, it is not surprising to find these elements in the tooth sample we studied. However, our main objective was to present a comparative study (comparing to a healthy subject) to investigate the accumulation of elements in tooth samples of renal patients.
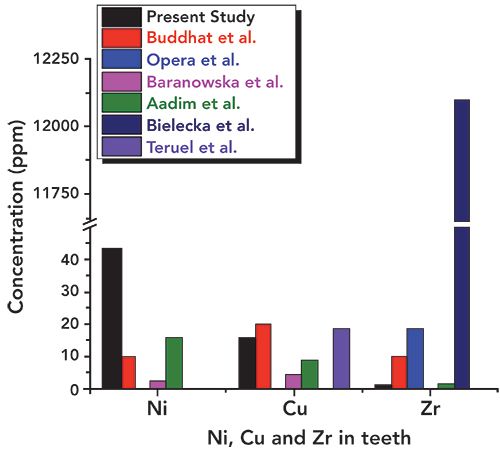
In Table I, we have summarized the concentrations of major and other heavy and toxic elements found in the tooth sample of the present study along with the literature reported values (16–21). We have considered the recent studies of tooth samples using XRF spectrometry alone. Figures 6–11 show the relative concentrations of different elements and their variations. The Ca to P ratio (Ca/P) is an indicator of the relative strength of bones or teeth (30,31). From the Ca/P ratio plotted in Figure 6, one can easily see that the Ca and P values measured from the sample are relatively less as compared to the values reported by others like Buddhachat and associates (16), Oprea and colleagues (17), Baranowska and co-workers (18), Aadim and associates (19), Teruel and colleagues (20), and Bielecka and co-workers (14). Also, the Ca/P ratio reported by Buddhachat and associates (9), Oprea and colleagues. (10), Baranowska and co-workers. (18), Aadim and associates (19), and Teruel and colleagues (20) lie in the range of 1.94 to 2.44, which is also in good agreement with the reported values (average value ~2.07) for sound enamel and dentin, with the exception of Bielecka and co-workers (21) (value ~1.77). In the present study, the value obtained was ~1.84, which indicates different Ca and P levels due to the CKD affected tooth. The major elements Mg, Na, and Cl were also found to be lower in the tooth sample as compared to the reported values (16–21), which can be seen in Figures 7 and 8.
Figure 6: Concentration of Ca and P within the tooth sample used in the present study as compared to literature values.
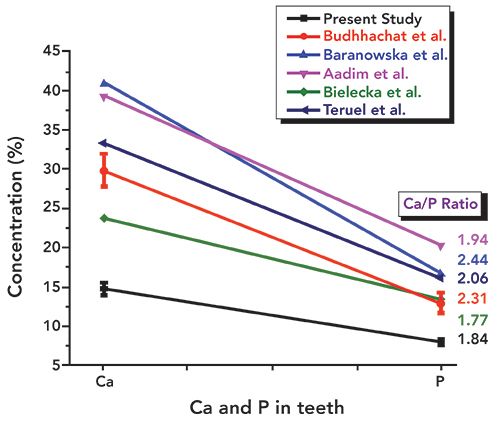
Figure 7: Relative concentrations of Mg and Na within the tooth sample used in the present study as compared to literature values.
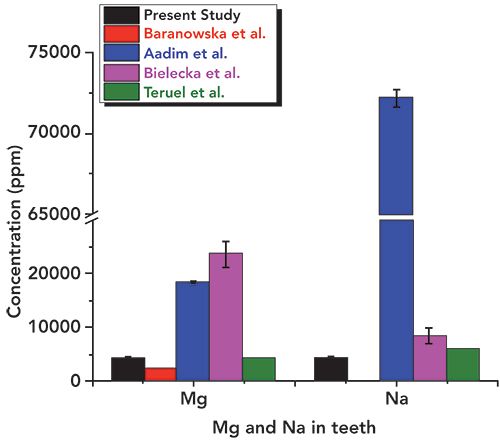
Figure 8: Relative concentrations of Cl, K, Fe, Al, Sr, and Cr in the tooth sample used for the present study as compared to literature values.
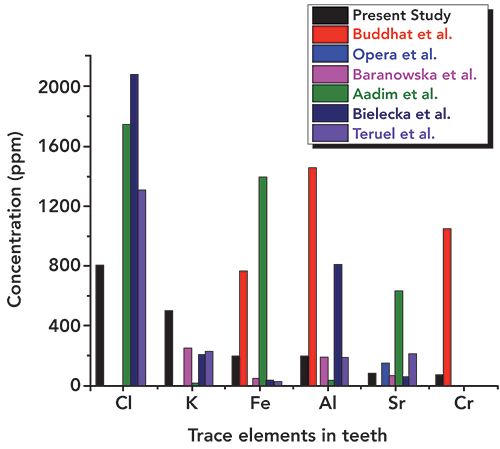
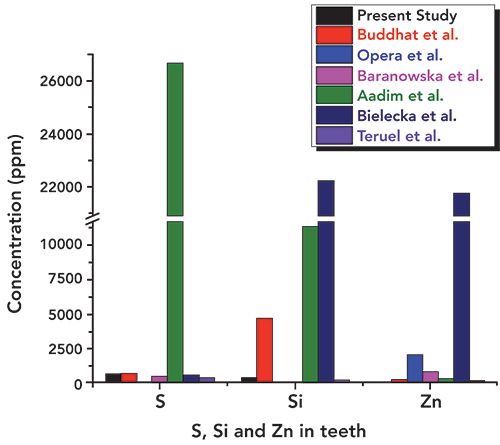
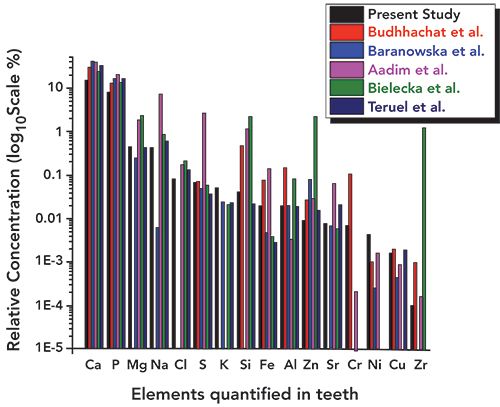
In addition, the concentration of essential elements such as S, Si, and Zn was observed to be lower (as can be seen in Figure 9) than the literature reported values (16–21). Values obtained for potassium (K) were a little higher (Figure 8) than those in the literature. The other heavy and trace elements such as Fe, Al, Sr, Ni, Cu, and Zr were found in a different concentration range, as shown in Figures 8–11. The overall relative concentrations in log percentage value of major, trace, heavy, and toxic elements found in the tooth sample in the present study and reported values are plotted for comparison. The relative difference in the concentration of elements, particularly Fe, Al, Sr, Ni, Cu, and Zr, may be due to the different environmental and geographical regions to which the patient belongs, and also the patient’s dietary habits, which is a major factor that can influence the concentration of such elements in teeth. Refer to references (16–21) for these elemental values.
Figure 9: Relative concentrations of S, Si, and Zn in the tooth sample used in present study and reported literature values.
Recently, Boskey (32) has discussed a variety of human diseases in which altered mineral properties occur in bones and teeth in relation to age and sex-matched healthy individuals, presenting some examples of the mineral changes that include frequently encountered diseases, such as osteoporosis and osteomalacia, and less-common diseases such as osteopetrosis, osteonecrosis, and osteogenesis imperfecta and odontogenesis imperfecta. Some of these diseases are due to genetic abnormalities, but they may also be attributed, to a varying degree, to environmental factors such as diet, exposure to sunlight, and exercise (33). Renal osteodystrophy was reported as a consequence of kidney malfunction, which leads to osteoporotic bone and decreased mineral contents (32−34). The decreased contents (as compared to healthy subjects) of essential elements, particularly Ca, P, Mg, Na, and Cl, in the tooth sample may also be attributed to the chronic kidney disease and hence may be related to renal osteodystrophy. The presence of other nonessential and toxic elements indicates their role in CKD and tooth mineralization. However, more elaborate experiments on multiple samples from different patients and their extensive studies will be needed.
Figure 10: Relative concentrations of Ni, Cu, and Zr in the tooth sample used in the present study and literature values.
The present study presents an interesting aspect for further studies of tooth samples of renal patients. In this case, we were only able to analyze the tooth sample from one CKD patient, as such tooth samples from renal patients (of different history) scarcely become available. We, therefore, have presented a case study only, on the elemental composition of a tooth sample from a renal patient. However, to extract detailed scientific data, more extensive studies will be needed to draw definite conclusions on the effects of CKD in calcified tissue materials like teeth and bones.
Figure 11: Overall relative concentrations (log10 scale) of major, trace, and heavy elements in tooth sample in the present study and literature values.
Conclusion
New spectroscopic and microscopic techniques are emerging every day, providing scientists with useful tools for characterizing even minute changes or transformations in crystal properties from diseases, providing potentially fruitful areas of collaboration between physicists and biologists and offering hope for the development of novel therapies. In the present study, we have successfully utilized WDXRF spectrometry for the detection and quantification of the elemental composition of a tooth sample from a renal patient, which indicates the potential usefulness of this technique toward the analysis of calcified tissue materials like bones and teeth. The elemental contents were compared with the literature values for a more fruitful discussion. The data demonstrated that the distribution of elements can be used to explain biological processes related to CKD. We have also utilized FT-IR as a powerful tool for detecting organic and inorganic constituents of biomaterials and successfully characterized phosphates, type A and B carbonates, amide, and water content in the tooth sample of the CKD patient. Therefore, our studies on the tooth sample demonstrate that FT-IR is a robust tool for detecting organic and inorganic constituents of biomaterials. The presence of variable contents of essential and nonessential elements indicates their role in CKD and renal osteodystrophy. However, more extensive spectroscopic investigations on tooth samples are required for more fruitful results using XRF spectrometry.
Acknowledgments
Both Ms. Shikha Sharma and Ms. Mahima Sharma both contributed equally in carrying out the experiments and drafting of the manuscript. We are thankful to the Sophisticated Analytical Instrumentation Facility (SAIF), at Panjab University, in Chandigarh, for providing WDXRF experimental facilities. Our special thanks go to Mr. Tejbir Singh at the WDXRF Lab SAIF, for his valuable suggestions and discussions related to WDXRF spectrometry and instrumentation. The authors are also thankful to staff members at the Department of Nephrology at Opal Hospital, in Varanasi, India, for their constant and timely support in planning the experiments on a sample of choice.
References
- C.J. Brown, S.R.N. Chenery, B. Smith, C. Mason, A. Tomkins, et al., Arch. Oral Biol. 49, 704−717 (2004)
- A. Belcourt and S. Gimleth, Calcif. Tissue Int. 28, 227−231 (1978).
- E. Sprawson and F.W. Bury, Proc. Royal Soc. Lon. B. Biol. 102, 419−426 (1928).
- M. Eskin, Vitam. Mineral 5, e149 (2016).
- B.G. Lowe, Nucl. Instrum. Methods A 439(2), 247−261 (2000).
- J.A. Cauley et al., Osteoporosis Int. 16, 1525−1537 (2005).
- B. Beckhoff, B. Kanngieber, N. Langhoff, R. Wedell, and H. Wolff, Handbook of Practical X-ray Fluorescence Analysis (Springer, Berlin, Germany, 2006).
- M. Haschke, Laboratory Micro-X-ray Fluorescence Spectroscopy (Springer International Publishing, Cham, Switzerland, 2014).
- R. Jenkins, R.W. Gould, and D. Gedcke, Quantitative X-ray Spectrometry (Marcel Dekker Inc., New York, New York, 1981).
- R.E. Van Grieken and A.A. Markowitz, Handbook of X-ray Spectrometry (Marcel Dekker Inc., New York, New York, 1992).
- The article can be found at: https//www.bruker.com/products/x-ray-diffraction-and-elemental-analysis/x-ray-fluorescence/s8-tiger/technical-details.html.
- The details can be found at: http://www.spectraplus.com/Downloads/htm.
- J. Sherman, Spectrochim. Acta7, 283−306 (1995).
- C.M. Zamudio-Ortega, R. Contreras-Bulnes, R.J. Scougall-Vilchis, R.A. Morales-Luckie, O.F. Olea-Mejía, and L.E. Rodríguez-Vilchis, European Journal of Paediatric Dentistry 15(3), 275−280 (2014).
- R.K. Ramakrishnaiah, G.U. Rehman, S. Basavarajappa, A.A. Al Khuraif, B.H. Durgesh, A.S. Khan, and I.U. Rehman, Appl. Spectrosc. Rev.50(4), 332−350 (2015).
- K. Buddhachat, S. Klinhom, P. Siengdee, J.L. Brown, R. Nomsiri, P. Kaewmong, et al. PLoS ONE11(5), e0155458 (2016).
- C. Oprea, P.J. Szalanski, M.V. Gustova, I.A. Oprea, and V. Buzguta, Vacuum83, S166−S168 (2009).
- I. Baranowska, L. BarchaÅski, M. BÄ k, B. Smolec, and Z. Mzyk, Pol. J. Environ. Stud.13(6), 639−646 (2004).
- K.A. Aadim, A.A.K.Hussain, and A.N. Ahmed, International Journal of Current Engineering and Technology6(6) (2016).
- J.D.D. Teruel, A. Alcolea, A. Hernández, and A.J.O. Ruiz, Arch. Oral Biol. 60, 768−775 (2015).
- K. Bielecka, W. Kurtek, D. Banas, A.K. Kukus, J. Braziewicz, U. Majewska, M. Pasek, J. Wudarczyk-Mocko, and I. Stabrawa, Acta Physica Polonica A125, 911−918 (2014).
- F. Brudevold, in Chemistry and Prevention of Dental Caries, R.F. Sognnaes, Ed. (Charles C Thomas, Springfield, Illinois, 1962), pp. 32.
- H. Leicester, Biochemistry of Teeth (C. V. Mosby Co., St. Louis, Missouri, 1949).
- C. Long, Ed., Biochemists’ Handbook (D. Van Nostrand Co., Inc., Princeton, New Jersey, 1961), p. 716.
- O.R. Trautz, Ann. NY Acad. Sci.60, 696 (1955).
- S.L. Rowles, in Structural and Chemical Organization of Teeth, Vol. 2, A.E. Miles, Ed. (Academic Press, Inc., New York, New York, 1967), p. 201.
- M.E.J. Curzon and J.D.B. Featherstone, in Handbook of Experimental Aspects of Oral Biochemistry, E.P. Lazari, Ed. (CRC Press, Boca Raton, Florida, 1983), p. 123-135.
- Q.Y. Ma, S.J. Traina, T.J. Logan, and J.A. Ryan, Environ. Sci. Technol. 28, 1219−1228 (1994).
- Q.Y. Ma, T.J. Logan, S.J. Traina, and J.A. Ryan, Environ. Sci. Technol.28, 408−418 (1994).
- M. Dermience, G. Lognay, F. Mathieu, and P. Goyens, J. Trace Elem. Med. Biol.32, 86−106 (2015).
- M.A. Amr , IJPS 6, 6241−6245 (2011).
- A.L. Boskey, Elements 3, 387−393 (2007).
- S.H. Ralston and B. de Crombrugghe, Genes Dev.20, 2492−2506 (2006).
- C.P. Sanchez, Peritoneal Dial. Int.26, 43−48 (2006).
Vivek K. Singh, Shikha Sharma, Mahima Sharma, Neha Sharma, and Jitendra Sharma are with the School of Physics at Shri Mata Vaishno Devi University, in Jammu and Kashmir, India. Pradeep K. Rai is with the Department of Nephrology at Opal Hospital, in Uttar Pradesh, India. Direct correspondence to: vivekksingh2005@gmail.com

Measurement of Ammonia Leakage by TDLAS in Mid-Infrared Combined with an EMD-SG Filter Method
April 9th 2024In this article, tunable diode laser absorption spectroscopy (TDLAS) is used to measure ammonia leakage, where a new denoising method combining empirical mode decomposition with the Savitzky-Golay smoothing algorithm (EMD-SG) is proposed to improve the signal-to-noise ratio (SNR) of absorbance signals.