The Benefits of Ion–Molecule Chemistry for the Determination of Titanium in Whole Blood and Serum Using Quadrupole-Based Collision–Reaction Cell ICP-MS Technology
This article highlights the benefits of using ion-molecule chemistry in a quadrupole-based collision–reaction cell to determine titanium levels in the presence of problematic polyatomic spectral interferences encountered in the analysis of human blood and serum samples from patients who have undergone artificial joint replacement surgery.
This article highlights the benefits of using ion-molecule chemistry in a quadrupole-based collision–reaction cell to determine titanium levels in the presence of problematic polyatomic spectral interferences encountered in the analysis of human blood and serum samples from patients who have undergone artificial joint replacement surgery. The study specifically focuses on the ability of ammonia gas to react with titanium in the cell to form an interference-free titanium molecular ion, which can then be used for quantitation. Detection limits and spike recovery data on serum reference materials will be presented that suggest that this methodology offers a viable alternative to double focusing, magnetic sector inductively coupled plasma–mass spectrometry technology for this analysis.
Titanium metal and its alloys are commonly used in metallic knee and hip replacement joints. Wear and tear over time often can cause metal particulates from the joint to contaminate the surrounding tissue. In addition, various electrochemical and corrosion processes taking place in the body can lead to serious leakage of titanium into the bloodstream. As a result, it is important to be able to monitor titanium levels in whole blood and serum in patients that have undergone, or are due to undergo, knee or hip replacement surgery. Unfortunately, the determination of titanium in these kinds of biological matrices by quadrupole-based inductively coupled plasma–mass spectrometry (ICP-MS) involves many challenges because of significant spectroscopic interferences from isobaric overlaps from calcium isotopes and other molecular ions; these interferences include ions such as PO+, SO+, CO2+, ArC+, NO2+, and ClC+ produced by a combination of matrix and solvent components in the sample. As a result, real-world detection capability by this technique is severely compromised (1). Table I provides a summary of these potential problematic polyatomic spectral interferences on the five titanium isotopes.
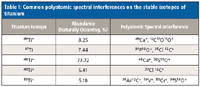
Table I: Common polyatomic spectral interferences on the stable isotopes of titanium
Moreover, interference-reduction techniques using collision–reaction cell technology have also proved inadequate to quantify titanium in these matrices because of the difficulty to reduce these problematic spectral interferences (2). Consequently, achievable titanium detection limits in these samples using quadrupole ICP-MS have been on the order of ≥200 µg/L (1,3). For this reason, researchers have turned their attention to magnetic sector ICP-MS to resolve the polyatomic interferences away from the analyte mass (4,5). This technique offered the potential of much lower detection limits, but in practice, proved to be somewhat problematic, because the only practical isotope available for quantitation was 47Ti (7.4% abundant) as a result of the interferences from 48Ca+ and 32S16O+ on the major isotope of 48Ti (73.7% abundant). In addition, to achieve satisfactory separation of 47Ti from the 31P16O+ interference, medium resolving power (Rs = 4000) was required, which also compromised the achievable detection capability. However, the instrument detection limit (IDL) and method detection limit (MDL) values obtained in one of these studies (5) were 0.01µg/L and 0.07 µg/L, respectively, which are still significantly better than those achieved using quadrupole ICP-MS.
Methodology
The aim of this study was therefore to evaluate the capabilities of ICP-MS with a quadrupole-based collision–reaction cell to reduce the impact of the interferences mentioned on the determination of titanium in whole blood and serum samples. The study used a NexION 300D ICP-MS system (PerkinElmer Inc.) fitted with a universal collision–reaction cell. In particular, the investigation evaluated the quality of the generated data by determining trace levels of titanium in Seronorm serum and whole blood standard reference (Sero AS, Norway) together with spike recoveries on real patient endogenous blood plasma and hip aspirate samples.
The interference removal capability and flexibility of the universal cell approach is well documented in the literature (6). Besides being used as a standard ICP-MS system, it also can be operated as a kinetic energy discrimination (KED)-based collision cell using inert gases such as He or as a dynamic reaction cell by using the most appropriate reaction gas for the efficient targeting of any spectral interference.
Traditionally, a quadrupole-based dynamic reaction cell is used to remove or reduce the impact of a polyatomic interference by the process of ion–molecule chemistry so the interference-free analyte mass can be used for quantitation. The advantage of using a quadrupole in the reaction cell is that the stability regions are much better defined than higher-order multipoles, so it is relatively straightforward to operate the quadrupole inside the reaction cell as a mass or bandpass filter and not just as an ion-focusing guide. Therefore, by careful optimization of the quadrupole electrical fields, unwanted reactions between the gas and the sample matrix or solvent that could potentially lead to new interferences are prevented. It means that every time an analyte and interfering ions enter the collision–reaction cell, the bandpass of the quadrupole can be optimized for that specific problem and then changed on the fly for the next one (7).
However, by selection of the optimum gas and gas flows, reactive gases can be also used to react with the analyte to produce a molecular ion that moves the analyte mass away to a higher mass region, which is free of the original polyatomic interferences. In the case of titanium in biological samples, the major isotope of titanium at m/z 48 can be moved to a higher mass by using ammonia (NH3) as a reaction gas in the cell to produce the 48Ti14NH(14NH3)4+ molecular ion (8), which can then be used for quantitation. This is exemplified in Figure 1, which shows a spectral scan at m/z 129–134 for 10 ppb Ti in reaction mode using an NH3 gas flow of 0.9 mL/min and a cell dynamic bandpass RP (q) tuning value of 0.2. It can be clearly seen that the isotopic fingerprint of the molecular ions approximates with the isotopic abundances of the five major titanium isotopes at m/z 46, 47, 48, 49, and 50.
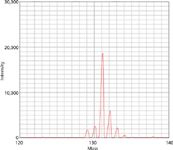
Figure 1: Scan of m/z 129â134 for 10 ppb Ti in reaction mode using an NH3 gas flow of 0.9 mL/min and a cell RP (q) value of 0.2.
The formation of this ionic complex was very stable and reproducible, as demonstrated by Figure 2, which shows a calibration curve for 0, 0.2, 0.5, and 1.0 µg/ L Ti at m/z 131.
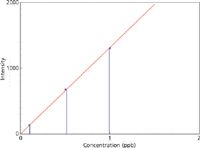
Figure 2: Calibration curve for 0, 0.2, 0.5, and 1.0 µg/L Ti at m/z 131.
It is also important to mention that xenon has a major isotope at m/z 131 (21.2% abundant), which could potentially interfere with the determination. However, because the ionization potential of NH3 (10.2 eV) is low compared to that of Xe+ (12.1 eV), the reaction is extremely exothermic and fast, with a rate constant of 10-10 cm3 molecule-1s-1. This means the Xe+ ion is converted to a neutral Xe species by a charge exchange mechanism and as a result does not interfere with the titanium determination. This reaction mechanism is shown by the following equation:
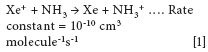
Instrumental Conditions
The ICP-MS instrumental conditions for this study are shown in Table II.
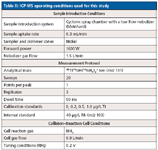
Table II: ICP-MS operating conditions used for this study
Sample Preparation
For the serum reference material, a simple 10-fold dilution using 0.1% nitric acid was used, whereas for the whole blood, endogenous plasma, and hip aspirate samples, 50 µL of sample and 50 µL of 200 µg/L of Rh internal standard were made up to 2.5 mL with deionized water. This represented a 50-fold dilution of the sample.
Results
Using matrix-matched calibration standards described previously, titanium was measured in Seronorm Trace Elements Serum L-1 and Seronorm Trace Elements Whole Blood L-1 and L-2 reference materials. The results obtained for both types of reference standards are shown in Table III.

Table III: Quantitative data for the determination of titanium in serum and whole blood reference standards
The method was used to measure titanium levels in patient samples and then spiked with known concentrations of titanium to evaluate spike recovery data. Two different samples were assessed — an endogenous blood plasma, which is blood taken from the joint area, and endogenous aspirate, which is fluid drained from an infected joint. The results and spike recoveries are shown in Table IV.

Table IV: Titanium spike recoveries for an endogenous blood plasma and hip aspirate
To get a better understanding of the detection capability of the serum method, 10 repeat measurements of a blank containing 10 µg/L calcium (to simulate the Ca levels in 10× diluted serum) were performed. The observed concentration in the matrix was 0.031 ± 0.00122 µg/L. This result suggests that a 3-sigma instrument detection limit of 0.0037 µg /L in solution (0.04 µg/L in serum) and a 10-sigma limit of quantification (LOQ) of 0.0122 µg/L in solution (0.12 µg/L in serum) are achievable.
Summary
This article has highlighted the benefits of using ion–molecule chemistry in a collision–reaction cell to move an analyte mass away from polyatomic spectral interferences that negatively impact its quantitation. Specifically, it has demonstrated the use of ammonia gas in a quadrupole-based collision–reaction cell to generate an interference-free titanium molecular ion in the determination of titanium in complex whole blood and serum samples. The achievable titanium detection limit of 0.04 µg/L in the serum matrix has also confirmed that this approach can realistically compete with the detection capability of high-resolution, magnetic sector technology for this application. The study has also demonstrated that the method is extremely robust by achieving accurate data with the serum reference materials and good spike recoveries with endogenous plasma and hip aspirate samples.
References
(1) D. Rodriguez, F.J. Gil, J.A. Planell, E. Jorge, L. Alvarez, R. Garcia, M. Larrea, and A. Zapata, Journal of Materials Science: Material in Medicine 10, 847–851 (1999).
(2) J.C. Rubio, M.C. Garcia-Alonso, C. Alonso, M.A. Alobera, C. Clemente, L. Munera, and M. Escudero, Journal of Materials Science: Material in Medicine 19(1), 369–375 (2008).
(3) S. McGarry, S.J. Morgan, R.M Grosskreuz, A.E. Williams, and W.R. Smith, Journal of Trauma and Acute Care Surgery 64(2), 430–433 (2008).
(4) A.S. Gonzalez, J.R. Encinar, J.M. Marchante-Gayon, and A. Sanz-Medel, Anal. and Bioanal. Chem. 393(1), 335–343, (2009).
(5) A.S. Gonzalez, J.M. Marchante-Gayon J.M. Tejerina-Lobo, J. Paz-Jimenez, and A. Sanz-Medel, Anal. and Bioanal. Chem. 391(7), 2583–2589 (2008).
(6) "Thirty-Minute Guide to ICP-MS," PerkinElmer Inc., Technical Note, 2011, http://www.perkinelmer.com/PDFs/Downloads/tch_icpmsthirtyminuteguide.pdf
(7) S.D. Tanner and V.I. Baranov, Atomic Spectroscopy 20(2), 45–52 (1999).
(8) York University, Ion Chemistry Laboratory Centre for Research in Mass Spectrometry, http://www.chem.yorku.ca/profs/bohme/research/element/Ti.html
David Price is PerkinElmer's ICP-MS product and applications specialist, based in the UK. Fadi Abou-Shakra is PerkinElmer's applications support leader for Northern Europe, based in the UK. Lynne Jung and Christine Sieniawska are advanced biomedical scientists in the Trace Element Unit of Southampton General Hospital in the UK. Robert Thomas runs his own scientific consulting company in Gaithersburg, Maryland, which focuses on trace element analysis. Direct correspondence to: david.price@perkinelmer.com

Inside the Laboratory – The Petrochronology Group at University of California, Santa Barbara
January 29th 2024In this edition of “Inside the Laboratory,” John Cottle, PhD, a professor of geology at the University of California, Santa Barbara, and a member of Spectroscopy’s Editorial Advisory Board, discusses his group’s most recent work using “laser ablation split steam” analysis to measure elemental concentrations and isotopic ratios in rocks and minerals.
Using ICP-MS to Advance the Work of the CDC
January 17th 2024At the Winter Conference on Plasma Spectrochemistry, Robert L. Jones, who recently retired from the Centers for Disease Control and Prevention (CDC), discussed his career at the CDC, and how his work with inductively coupled plasma–mass spectrometry (ICP-MS) assisted in addressing pivotal public health crises.