Detection and Sourcing of Counterfeit Pharmaceutical Products and Consumer Goods
Special Issues
The authors discuss the use of vibrational spectroscopy to differentiate an authentic article from a counterfeit one throughout a product's lifecycle, from component receipt at the site of manufacture, to product receipt by the end user.
The authors discuss the use of vibrational spectroscopy to differentiate an authentic article from a counterfeit one throughout a product's lifecycle, from component receipt at the site of manufacture, to product receipt by the end user.
While the challenge of facing this type of illicit activity might seem overwhelming for a variety of reasons, the need to do so has never been more critical. Aside from the loss of revenue, there are other considerations that are just as significant. Many of these counterfeit products pose a significant threat to health and safety due to substandard quality control, and some have even been shown to be harmful to the user (2). Counterfeiting and piracy also have a long-term negative impact on innovation and brand recognition, and channel a considerable portion of revenue to criminal networks, organized crime, and other groups that disrupt and corrupt society (3). In addition, intellectual property in the form of copyrights, patents, and trademarks promotes cultural development, fosters innovation and growth, and protects public health and safety (4). Theft of these goods affects all industry sectors and results in substantial economic, social, and developmental costs that have increased exponentially in recent years. Piracy and counterfeiting have been shown to decrease employment opportunities, diminish tax revenues for governments, and reduce incentives to invest (5).
Product Authentication Using Analytical Chemistry
Defense of intellectual property assumes that it is possible to differentiate an authentic article from a counterfeit one. Frequently this is a relatively simple task. As counterfeiters become more experienced in their trade, though, differentiation becomes more and more challenging. In many instances, it is necessary to perform chemical analysis before a conclusion about authenticity can be made. In addition, due to the complex nature of the lifecycle of a counterfeit article, it has become necessary for brand owners across many different industries to employ methods for detection and authentication at various levels within their legitimate supply chains. For instance, in the pharmaceutical industry, many companies employ in-house forensic scientists to address product authentication concerns. Within the textile industry, safeguarding the integrity of natural organic fibers is an absolute requirement, as contamination with pesticide-tainted counterfeits undermines quality and, ultimately, revenue. In the electronics industry, counterfeiting has been a problem for a long time, but the current economic challenges appear to be making things even worse. The industry has even begun to adopt practices like qualifying reliable vendors to help prevent counterfeits from entering their supply chain and marketplace. Although this is a common practice in the pharmaceutical industry, it has been adopted only recently within the electronics arena.
The level of testing performed to determine authenticity is variable, but there are analytical methods known to be effective at providing proven results that enable effective chemical differentiation. For instance, vibrational spectroscopy in the form of both infrared (IR) and Raman spectroscopy has been used with success to perform chemical identifications on both the counterfeit article itself and on the packaging used for transport of the article (6). Figure 1 shows the IR spectra collected from packaging from genuine and replicate medications. These spectra show that the chemical information contained within an IR spectrum frequently can be quickly and easily (within seconds) used to differentiate the coating layer of packaging used. Differences between these two samples are evident across the range of the mid-IR spectrum. Analysis was performed using the IdentifyIR portable Fourier-transform infrared (FT-IR) spectrometer (Smiths Detection, Alcoa, Tennessee).
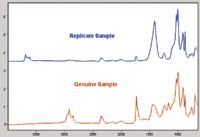
Figure 1: Infrared spectra collected from the outside packaging of genuine and replicate samples.
Vibrational microspectroscopy also can be used to differentiate components of the counterfeit product itself. Figure 2 shows the IR spectrum and photomicrograph collected from a cross-section of a genuine tablet, as well as the IR spectrum of a known reference standard of pseudoephedrine hydrochloride. These data show that the active pharmaceutical ingredient can be selected for microspectroscopic analysis using polarized light microscopy or other contrast-enhancement techniques. The coupling of the microscope with microspectroscopy makes it possible to collect spectra from discrete locations within a relatively complex matrix. In this instance, IR microspectroscopy was used to perform the chemical identification using an IlluminatIR IR microprobe (Smiths Detection). This IR microprobe allows the user to employ contrast-enhancement methods such as polarized light or fluorescence microscopy to visualize the sample and selectively isolate particles for microspectroscopic identification. It is also possible to use Raman microspectroscopy to perform a similar type of chemical analysis. Frequently, although not always, the chemical composition of counterfeit samples will not only have different excipients (nonactive components) from the replicate sample, but it might not even contain the active pharmaceutical ingredient at all (7).

Figure 2: Infrared spectrum showing the active pharmaceutical ingredient contained within tablet cross-section.
Product Authentication and Brand-Management Protection
Advances in technology make it possible to perform these and other types of chemical analysis, but protection of brand owner rights is not simply based upon detection of a counterfeit article when one presents itself. It relies upon maintaining control throughout a product's lifecycle. This can be a very difficult challenge, especially considering the global economy within which many commercial products are manufactured. Products frequently contain components from around the world, regardless of where final assembly or manufacture of the product might occur. The ability to establish control can be challenging in the best of circumstances and is almost always difficult to manage. The IntelliMark Solution product identification system (Smiths Detection in partnership with ARmark Authentication Technologies, LLC, Glen Rock, Pennsylvania) enables control and authentication of both the components used during manufacture or assembly and of the finished product. Product authentication can be verified anywhere along its lifecycle.
The product identification system is a complex, customized product authentication solution that makes it possible for the brand owner to establish and maintain control by building it in at the beginning of the product's lifecycle. Information-laden, custom-designed covert markers are either incorporated into the raw materials and components, or they can be placed within the final product or its packaging and shipping containers. Product authentication can occur in transit or when the product is delivered to its final destination. The industries within which this technology can be applied effectively are virtually unlimited, ranging from the food industry, where the covert markers are manufactured using ingredients that are generally recognized as safe (GRAS) (8) for human consumption, to the electronics industry, where even fluorescent or other types of chemically elegant markers can be designed and used.
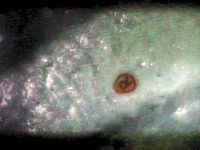
Figure 3: Photomicrograph of covert marker (approximately 75 μm) in the finished product for detection in transit or at final destination.
Regardless of the industry served, the markers are detected easily using instruments designed to be used in the environments encountered along the supply chain. Figure 3 shows a covert marker (ARmark Authentication Technologies, LLC) detected using the IdentifyIR (Smiths Detection) spectrometer. This instrument is used in the laboratory or on the loading dock and is capable of both creating a visual image of the covert marker and collecting an IR spectrum for chemical identification of either the marker, the product, or its packaging or shipping container. Both the image and the IR spectrum can be used to set pass–fail criteria for authentication, which can be established as part of an interactive service between the brand owner and Smiths Detection. This interactive service is a critical component of the product identification system. The spectrum of the covert marker shown in Figure 3 as well as of the matrix within which the marker is incorporated is shown in Figure 4.

Figure 4: Infrared spectrum of covert marker (red) and of the matrix (blue).
Alternate wavelength light-source accessories can be used to help locate the covert markers when the markers are fluorescent, and also can serve as an additional level of security for authentication of the covert marker. Figure 5 shows the polymer matrix of an electronics board impregnated with covert markers. A handheld ultraviolet light source was used to induce fluorescence at the time this photomicrograph was collected. The type of covert marker offered is customized to meet the needs of the customer and can be simple or complex, depending upon the user's needs.

Figure 5: Covert markers dispersed within polymer matrix visualized using an ultraviolet light source accessory with a digital microscope.
Summary
In recent years, counterfeiting, piracy, and other intellectual property rights violations have grown in magnitude and complexity, costing businesses billions of dollars in lost revenue and often posing health and safety risks to consumers. This growth was fueled in part by the spread of enabling technology, allowing for simple and low-cost duplication of copyrighted products, as well as by the rise in organized crime groups that smuggle and distribute counterfeit merchandise for profit (9). Global access to the Internet also plays a critical role, especially with regard to accessibility to counterfeit pharmaceuticals. The Internet has become the primary tool for criminal organizations to advertise, communicate, and conduct sales of counterfeit pharmaceuticals, as well as being the primary mechanism for consumers to find, order, and make payments for counterfeit pharmaceuticals (10). Considering these and other facts, brand owners need to be vigilant about how they protect their intellectual property. Building control into a product's lifecycle provides the greatest level of security. These combined capabilities enable detection and authentication throughout a product's lifecycle from component receipt at the site of manufacture, to product receipt by the end user.
John A. Reffner is with John Jay College of Criminal Justice, CUNY, New York, New York. Peter D. Gabriele is with ARmark Authentication Technologies, LLC, Glen Rock, Pennsylvania. Pauline E. Leary is with Smiths Detection, Alcoa, Tennessee.
References
(1) The Economic Impact of Counterfeiting and Piracy, Executive Summary, 2008, www.oecd.org.
(2) http://www.ul.com/newsroom/anticounterfeiting/risk.html.
(3) The Economic Impact of Counterfeiting and Piracy, Executive Summary, 2007, www.oecd.org.
(4) http://usinfo.state.gov/products/pubs/intelprp/protecting.htm.
(5) http://www.iccwbo.org/policy/ip/id2948/index.html.
(6) Smiths Detection Application Brief AB-83, Trace Evidence Analysis Using the IdentifyIR Diamond ATR Spectrometer.
(7) http://www.usdoj.gov/dea/programs/forensicsci/microgram/mg0106/mg0106.html.
(9) http://www.ice.gov/pi/cornerstone/ipr/index.htm.
(10) ICE Efforts to Combat Counterfeit Pharmaceuticals, Fact Sheet, July 11, 2006.
