The Determination of 226Ra in Nontypical Soil Samples by ICP-MS
This article describes a way to measure 226Ra using inductively coupled plasma–mass spectrometry (ICP-MS) rather than the conventional method of gamma spectroscopy, taking into account an undocumented interference (207Pb19F) that is caused by the requirement to use hydrofluoric acid during sample preparation. The unusually high Pb levels observed in a number of soil samples caused significant interferences at the very low concentrations of 226Ra that were measured. The expected 208Pb18O interference was insignificant under optimized instrument conditions.
This article describes a way to measure 226Ra using inductively coupled plasma–mass spectrometry (ICP-MS) rather than the conventional method of gamma spectroscopy, taking into account an undocumented interference (207Pb19F) that is caused by the requirement to use hydrofluoric acid during sample preparation. The unusually high Pb levels observed in a number of soil samples caused significant interferences at the very low concentrations of 226Ra that were measured. The expected 208Pb18O interference was insignificant under optimized instrument conditions.
As part of a remediation project, soils were analyzed for a list of elements of interest, plus a number of specific isotopes. Inductively coupled plasma–mass spectrometry (ICP-MS) has seen increasing use for the determination of long-lived isotopes in preference to traditional counting techniques because of various efficiencies. In this study, 230 Th and 226Ra were of specific interest. Typically, 226Ra has been determined using gamma spectroscopy. However, determination by gamma spectroscopy requires equilibration of the sample before final determination can be made, and full equilibration can require up to 30 days. Despite this, the initial phases of the remediation work did not allow for such a long delay period, hence ICP-MS was considered as a more rapid evaluation tool. Without this equilibration, there is danger of producing a significant low bias if gamma counting is used, which is a major factor if a reduction in equilibration times is considered. In matters of health and safety, it is always prudent to choose a method with a potential positive bias rather than one with a negative bias. Hence, it was decided that an ICP-MS method should be developed to speed up the determination of 226Ra in soil samples instead of simply reducing the equilibration period before gamma counting.
It became apparent during the analysis of these samples that they were not typical environmental soils, but more like mine tailings because of the mining and smelter activities over the last several decades in the area they were taken from. Besides having concentrations of many elements that exceeded environmental regulations in Ontario, the lead content in the soils caused an interference on the 226Ra determination by ICP-MS. The known Pb oxide (208 Pb18 O) interference on 226Ra was minimized by optimizing instrument conditions so as to have low oxide formation (<3%). The overall impact was therefore found to be insignificant in all of the samples examined.
However, an undocumented interference was caused by the requirement to use hydrofluoric acid (HF) in the sample preparation to get a complete digestion. The interference caused by 207 Pb19F, although much greater than that due to oxide formation, is not significant for normal soils. The unusually high Pb levels observed in a number of soil samples caused significant interferences at the very low concentrations of 226Ra being measured. This problem led to the present study.
Experimental
Sample Preparation
Soil samples were dried in ovens at 40 °C for approximately 4–6 h to remove any moisture from the soil without a significant loss of volatiles that were of interest for other measurements. The drying time varied because of the fact that some of the soil samples were extremely wet and contained clay-like material that took a longer time to dry than the other soils. After they were dried, the samples were pulverized and homogenized before being passed through 50 mesh sieves. A representative 0.5-g portion of the dried homogenized samples was digested using a CEM Mars 5 microwave (CEM Corporation, Matthews, North Carolina) using 10 mL of a mixture of ultrapure HNO3, HCl, and HF (8:2:1, respectively). After they were digested and cooled, the samples were quantitatively transferred to clean Environmental Express (Charleston, South Carolina) disposable digestion tubes and bulked to 50 g using >18-MΩ/cm2 water. This represented the "base" working concentration for samples. Appropriate quality control samples were included in the digestion, including acid blanks, sample duplicates, and certified reference materials (contaminated soil SS-2 from SCP Science [Baie D'Urfé, Quebec, Canada], NIST SRM 1645 river sediment, or NIST SRM 1646).
Analysis
Samples were analyzed for 232 Th, As, Sb, Co, Cu, Ni, U, Pb, Ba, Be, B, Cd, Hg, Mo, Se, Ag, V, and Zn using the PerkinElmer (Waltham, Massachusetts) Sciex Elan 6100 DRC ICP-MS system. All samples were diluted a further 10 times from the "base" concentration with >18-MΩ/cm2 water before analysis to minimize both the acid content and the total dissolved solids present in the samples introduced to the ICP-MS. Where possible, multiple isotopes of an element were analyzed to ensure that interferences were not present. The ICP-MS system was calibrated every analytical run using second source standards for quality control standards and internal standardization (with 100 ppb rhodium added using a second channel on the peristaltic pump) to compensate for instrument drift. All standards were matrix-matched to the acid content of the digested and diluted samples.
Samples were also analyzed for 230 Th, and 226Ra using a Varian (Palo Alto, California) 820-MS ICP-MS system. All samples were diluted four times from the "base" concentration with >18-MΩ/cm2 water before analysis to meet the required detection limits for these isotopes. Although this was not an ideal situation, the physical stability of the instrument because of its cone design and 90° reflecting ion optics system (1) allowed for smaller dilution to be used. The ICP-MS system was calibrated every analytical run using second source standards for quality control standards. All standards were matrix-matched to the acid content of the prepared samples. A matrix-matched acid blank was analyzed after every sample to rinse out the system, and the instrument was recalibrated after the analysis of every five samples.
Both ICP-MS instruments were optimized daily before sample analysis to ensure that instrument conditions achieved maximum sensitivity, low background, low oxides (<3%), and low doubly charged species (<3%).
226Ra and 207Pb19F Study
Standards Preparation
Standards of varying concentrations of lead and HF were prepared to determine the effect of the 207Pb19F interference on 226Ra. These standards were made using a commercially available single-element lead standard with natural abundance isotope distribution from Inorganic Ventures (Christiansburg, Virginia). The standard was first diluted to produce a 100 ppm intermediate lead standard and finally to the desired working concentrations required for the study.
The first step was to fix the amount of HF at 1% by weight while varying the concentration of total lead in the standards. The lead standards used were 0.2, 0.4, 0.6, 0.8, 1.0, 1.2, 1.4, 1.6, 1.8, 2, 3, 4, 5, 6, 7, 8, 9, 10, 12, 14, 16, 18, and 20 ppm. The lead standards were made gravimetrically, using the appropriate weight of 100 ppm intermediate standard, adding 1% high-purity HF, and bulking to the appropriate final weight using >18-MΩ/cm2 water.
The second step was to fix the amount of lead present at 20 ppm, while varying the concentration of HF in the standard. The concentrations of HF used (all in weight %) were 0.25, 0.5, 1, 2, and 3. Again, the standards were made gravimetrically using the appropriate weight of intermediate Pb standard, adding the appropriate amount of high-purity HF to reach the desired acid concentration, and bulking to the appropriate final weight using >18-MΩ/cm2 water.
Analysis of 207Pb19F Standards
Samples were analyzed for 226Ra and 207Pb using the Varian 820-MS ICP-MS system. The system was calibrated before analysis using second source standards for quality control standards. All standards were once again matrix-matched to the acid content of the digested samples that would be analyzed using this method.
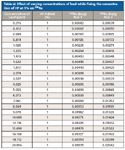
Table sI: Effect of varying concentrations of lead while fixing the concentration of HF at 1% on 226Ra
The ICP-MS instrument was optimized daily before sample analysis to ensure that instrument conditions achieved maximum sensitivity, low background, low oxides (<3%), and low doubly charged species (<3%).
Results and Discussion
Table sI (available online) and Figure 1 show the results of the first step of the study, looking at varying concentrations of lead while fixing the concentration of HF at 1%. As seen in these results, the higher the concentration of Pb, the greater the 207Pb19F interference on 226Ra. This effect had been noted when comparing the ICP-MS data to that found by gamma spectroscopy. The second step of the study looked at varying the concentration of HF while keeping the concentration of lead constant. These results are shown in Table sII (available online) and Figure 2. These data show that there is not a direct linear relationship between the concentration of lead and HF in the sample. This is as expected for a moderate equilibrium (equilibrium constant has a moderate value).

Figure 1: Effect of varying concentrations of Pb while fixing the concentrations of HF at 1% on 226Ra.
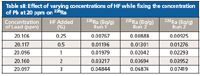
Table sII: Effect of varying concentrations of HF while fixing the concentration of Pb at 20 ppm on 226Ra
Table sIII (available online) and Figure 3 show the 226Ra comparison results between analysis by gamma spectroscopy and ICP-MS (both with and without the correction factor for lead concentration). It is important to note that the gamma data were determined on samples upon arrival at the laboratory, based on 214Pb and 215Bi analysis, and will be biased low. 226Ra concentrations are expected to increase during the 30-day in-growth period required for this analysis by gamma spectroscopy. This is clearly seen in samples containing higher concentrations of 226Ra, in which the initial gamma data are significantly lower than those found by ICP-MS using the determined correction factor. However, there is a good correlation between the two techniques when the correction factor is used, as illustrated.

Figure 2: Effect of varying concentrations of HF while fixing the concentration of Pb at 20 ppm on 226Ra.
Because a constant concentration of HF is generally used for sample digestion in a given study, a simple correction factor can be experimentally determined for any given level of HF rather than using a more complex correction equation. A complex correction equation also could be determined by a more extensive study of the Pb–F equilibrium under spectroscopic conditions. This factor was determined for the HF concentration that was used for the digestion of the soils for this remediation work: The more tightly experimental conditions are controlled (particularly the acid digestion), the more accurate the results of the correction factor. ICP-MS was the method of choice for the determination of 226Ra because of the required turnaround times for this project (24–48 h from date of receipt at the laboratory). As mentioned above, 226Ra determination by gamma spectroscopy requires an equilibration time of up to 30 days for the sample before final determination to avoid significant low bias. An extended commentary on radiochemical methods is required to explain why no viable alternatives existed using the gamma measurement method. In matters of health and safety, it is always prudent to choose a method with a potential positive bias rather than one with a negative bias. Therefore, to meet the required timelines, ICP-MS was used for 226Ra.
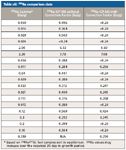
Table sIII: 226Ra comparison data
To further test the accuracy of the data produced using the ICP-MS method for the determination of 226Ra, the Kinectrics Inc. laboratory (Toronto, Ontario, Canada) participated in an interlaboratory study from Environmental Research Associates (ERA) in Arvada, Colorado (Study RAD-84). The value determined by the laboratory was 7.81 pCi/L 226Ra. The preliminary assigned value was reported as 8.26 pCi/L 226Ra; therefore the value determined using this method was well within the preliminary acceptance limits of 6.21–9.71 pCi/L 226Ra (2).
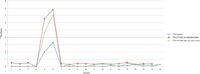
Figure 3: 226Ra (Bq/g) comparison.
Conclusion
The determination of 226Ra in soil samples in the presence of high concentrations of Pb was not a straightforward analysis. However, a correction factor was determined that could be applied to the 226Ra data to account for this interference. This correction factor worked well, as seen when 226Ra found by gamma spectroscopy was compared to the ICP-MS data, showing a very good correlation between the two independent techniques. Any additional method improvements would require chemical separations for sample cleanup before analysis or the use of a sector-field ICP-MS system to improve the distinction between species (hence smaller correction factor).
Teresa Switzer, Otto Herrmann, and Darko Ilic are with Kinectrics Inc., Toronto, Ontario, Canada. Direct correspondence to: Teresa.SWITZER@kinectrics.com.
References
(1) Varian 810-MS and 820-MS ICP Mass Spectrometers Specification Sheet, SI-0184 10/05.
(2) Environmental Research Associates Study RAD-84, February 2011.

Inside the Laboratory – The Petrochronology Group at University of California, Santa Barbara
January 29th 2024In this edition of “Inside the Laboratory,” John Cottle, PhD, a professor of geology at the University of California, Santa Barbara, and a member of Spectroscopy’s Editorial Advisory Board, discusses his group’s most recent work using “laser ablation split steam” analysis to measure elemental concentrations and isotopic ratios in rocks and minerals.