The Determination of Phosphorus, Sulfur, Sodium, Potassium, Calcium, and Magnesium in Biodiesel
The author looks at methods used to detect the presence of contaminants in biodiesel.
Biodiesel is a renewable fuel produced from vegetable oil, animal fats, and waste cooking oils. Feedstocks such as soy, canola, mustard, sunflower, coconut, palm, and cottonseed oil as well as beef tallow and fish oils have been used to manufacture biodiesel.
As a result of the world's energy demands, the use of biodiesel is increasing rapidly. Blends of biodiesel with conventional petroleum diesel represent a common use of biodiesel. In the United States, B20 (a blend of 20% biodiesel and 80% petroleum diesel) is recognized as an alternative diesel fuel. Biodiesel has a number of advantages, including:
- Reduction of toxic exhaust emissions — lower amounts of hydrocarbon (HC), carbon monoxide (CO), and particulate emissions (PM)
- Greenhouse gas savings — carbon dioxide (CO2) produced by burning biodiesel is used by subsequent crops used to produce the fuel
- Fast biodegradability — approximately 4–5 times as fast as petroleum diesel
- Lower toxicity than petrodiesel
- Higher flashpoint than petroleum diesel — 150 °C versus 70 °C
- Can be used in diesel engines with little or no modification
Because the presence of contaminants can lead to operational problems, the American Society for Testing and Material (ASTM) and European Standard (EN) have developed standards to which pure biodiesel (B100) can be tested. The ASTM Standard is D-6751, shown in Table I.
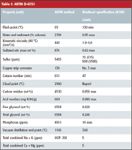
Table I: ASTM D-6751
Physical, chromatographic, and spectroscopic methods are used to apply the standard. Here we look at the use of the FuelPro Biodiesel Metals Analyzer (Teledyne Leeman Labs, Hudson, New Hampshire) to apply the ASTM standard.
Instrumentation
Accurate elemental analysis of biodiesel requires analytical methodology that is both sensitive and selective. The analyzer meets these requirements and can determine up to 70 different elements in a sample. The analyzer permits close monitoring of elemental content throughout processing, from the raw oil to the finished product. Trace metal analysis is an important part of quality control as well as quality checks of the finished products.
The effects of the biodiesel not meeting the specifications of the D-6751 standards follow:
- Phosphorus — Phosphorus has been shown to damage the ability of after-treatment systems to reduce exhaust emissions as intended. The influence of phosphorus is cumulative; as a result, very low levels of contamination over the significant amount of fuel consumed by an engine can lead to unexpected deterioration of the after-treatment system.
- Alkali and alkaline metals — Sodium and potassium hydroxides are utilized as catalysts, and magnesium and calcium as absorbents in the production of biodiesel and should be removed through the biodiesel production process. These residual metals can form deposits in fuel injection system components and poison emission control after-treatment systems.
- Sulfur — Sulfur levels in fuel are regulated by various governmental agencies to assure compatibility with emission standard requirements. In the U.S., there are currently three sulfur grades: S5000, S500, and S15, for both D1 and D2 petroleum diesel fuel. Biodiesel blends must not exceed the applicable maximum sulfur levels as defined for petroleum diesel.
Method
Biodiesel samples were prepared by diluting 1:10 with kerosene. The analyzer was calibrated with standards prepared by diluting Plasma-Pure biodiesel stock standards. Standard concentrations were at the 0.00, 10.0, and 20.00 ppm levels for Na, K, Cu, Mg, P, and S.
The analyzer's preprogrammed method was followed for the set-up and analysis of biodiesel.
Results
The values obtained as a result of the analysis of a B100 biodiesel sample are shown in Table II. All concentrations are given in ppm. The concentrations in the original sample are listed in the column labeled "Final Concentration (ppm)" ("ND" indicated that the analyte was not detected). The column labeled "Spike recovery (%)" contains the spike recovery data for a 3-ppm spike of all the analyte elements. (A separate sample was spiked with approximately 9 ppm sulfur to determine its recovery since the multielement stock standard contains sulfur as a matrix element.) The spike data show excellent recoveries, indicating that the 1:10 dilution is sufficient to eliminate any viscosity effects that may affect accuracy.
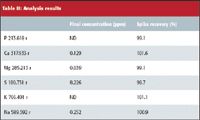
Table II: Analysis results
The results indicate that this biodiesel sample passes the ASTM D-6751 standard. The result for sulfur indicates that it successfully passes the S-15 low sulfur designation that all highway diesel fuels had to meet from 2007 onward.
Table III contains typical detection limits obtained in the oil matrix. The detection limits were determined by taking three replicates measurements in blank oil and multiplying the standard deviation by three.
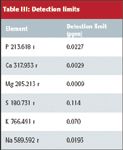
Table III: Detection limits
Conclusion
Biodiesel is analyzed effectively using this method, and the detection limit capability of the instrument meets the requirements of the ASTM and EN Standards against which biodiesel must be measured.
Samples also are prepared effectively by dilution with a suitable solvent. Excellent recoveries are obtained from spiked biodiesel samples. This indicated that the method is suitable for the analysis of biodiesel fuels and that matrix interferences are not a problem.
Manny Almeida is with Teledyne Leeman Labs, Hudson, New Hampshire.
References
(2) Specification for Biodiesel (B100) 0 ASTM D6751.

Inside the Laboratory – The Petrochronology Group at University of California, Santa Barbara
January 29th 2024In this edition of “Inside the Laboratory,” John Cottle, PhD, a professor of geology at the University of California, Santa Barbara, and a member of Spectroscopy’s Editorial Advisory Board, discusses his group’s most recent work using “laser ablation split steam” analysis to measure elemental concentrations and isotopic ratios in rocks and minerals.