Gaining Insight into Cocoa Butter Polymorph Formation Through In Situ Rheology and Raman Spectroscopy
Special Issues
The hyphenation of rheology and Raman spectroscopy enabled a more holistic depiction of the crystallization process and provided unique insights into the formation of cocoa butter polymorph form IV.
Rheology coupled with in situ Raman spectroscopy (rheoRaman) was used to examine the isothermal crystallization of cocoa butter. Two unique isolation protocols were used to form cocoa butter polymorphs form III and form IV. The hyphenation of these two independent analytical techniques, rheology and Raman spectroscopy, enabled a more holistic depiction of the crystallization process and provided unique insights into the formation of cocoa butter polymorphs.
Cocoa butter is an edible vegetable fat extracted from the cocoa bean. Cocoa butter is commonly used in home and personal care products (such as ointments and lotions) and it is a vital ingredient in chocolate. Cocoa butter forms the continuous phase within chocolate confections and is responsible for the chocolate's texture, snap, gloss, melting behavior, and resistance to fat bloom. These physical characteristics are a direct result of cocoa butter's triacylglycerol (TAG) composition and overall crystalline structure.
In general, TAG molecules assume a tuning fork configuration and the TAG "forks" assemble to form crystal lattice structures. During crystallization, the TAG molecules slow down as the cocoa butter oil cools and the TAGs come to rest in contact with one another, forming subcrystalline cells (1). After the subcells are formed, they are thermodynamically driven to aggregate into larger and more-stable crystalline structures (2). The self-assembly of subcell structures and their further aggregation is governed by a balance of intra- and intermolecular interactions. Depending on the molecular level packing and orientation of the TAGs, cocoa butter can form different types of crystal lattice structures, commonly referred to as polymorphs.
Researchers have identified six unique cocoa butter polymorphs (3)- form I, II, III, IV, V, and VI-each with a distinct molecular signature. Form V is the polymorph most commonly found in commercially available chocolates, and it is believed that all other polymorphs lead to inadequate properties or a shortened shelf life. For example, form IV is often associated with poorly or under-tempered chocolate; whereas form VI is considered to cause fat bloom, the greyish surface coating sometimes observed on old or temperature-abused chocolate. As a result, the chocolate tempering process is critical because it ensures that the cocoa butter has assembled into the desired crystalline polymorph.
Overall, cocoa butter crystallization is a highly complex, multistage process. Understanding the isothermal crystallization behavior of cocoa butter is vital for improving chocolate manufacturing processes and maintaining product quality. Cocoa butter crystallization has been widely studied using a variety of analytical techniques, including differential scanning calorimetry (DSC), X-ray diffraction (XRD), X-ray scattering, rheology, and Raman spectroscopy (2,4–7). Although cocoa butter crystallization has been thoroughly examined from the molecular, microstructural, and macroscopic levels, a lack of consensus about the existence of some cocoa butter polymorphs, the overall crystallization kinetics (especially in the presence of additives), and the underlying mechanisms of the crystallization process still persist.
In this study, rheology coupled with in situ Raman spectroscopy was used to examine the isothermal crystallization of cocoa butter. Raman spectroscopy is a highly sensitive, relatively fast, and nondestructive technique that can probe the molecular structure and conformation in both liquid and solid TAG assemblies, as well as intra- and inter-TAG interactions. With simultaneous Raman and rheological measurements, molecular-level interactions and conformational shifts during the isothermal crystallization of cocoa butter were directly correlated with the changes in bulk viscoelastic properties, providing unique insight into the multifaceted crystallization behavior of cocoa butter.
Materials and Methods
Materials
Commercially available, organic cocoa butter (Theobroma cacao) was acquired from Inesscents.
Rheology
Rheological measurements were performed using a Thermo Scientific HAAKE MARS 60 rheometer equipped with a serrated 35-mm-diameter plate rotor at a gap height of 1 mm. The serrated plate was used to prevent slip at the sample-rotor interface. All measurements were conducted using oscillatory shear, with a frequency of 1 Hz and a constant strain of 0.1%; data were collected every 10 s. Cocoa butter samples were loaded onto the rheometer at 60 °C and allowed to equilibrate for 10 min to erase any crystal structures or shear history from sample loading. After the equilibrium step, samples were exposed to one of the following temperature ramp and isothermal hold procedures to generate cocoa butter polymorph form III or form IV (2):
Form III (Slow Cooling)
- Temperature decreased from 60 °C to 14 °C at a rate of 2 °C/min
- Temperature held constant at 14 °C for 60 min
Form IV (Rapid Cooling)
- Temperature decreased from 60 °C to 22 °C at a rate of 10 °C/min
- Temperature held constant at 22 °C for 120 min
The melt-to-solid phase transition of cocoa butter was probed rheologically using small amplitude oscillatory shear measurements, where the storage modulus G' and loss modulus G" were measured as a function of time. The overall magnitudes of G' and G", as well as the ratio of G"/G' = tan(δ), determine the general viscoelasticity and overall resistance to deformation for a given material. The term "tan(δ)" is also referred to as the loss or damping factor where δ is the phase angle defined as the shift or lag between the input strain and resultant stress sine waves (or vice versa) during an oscillatory shear measurement. Values of tan(δ) less than unity indicate elastically dominant (solid-like) behavior, whereas values greater than unity indicate viscously dominant (liquid-like) behavior. Unlike the individual moduli, tan(δ) can be used to quantify overall brittleness of a material and is commonly used to assess glass transition behavior. In general, as tan(δ) becomes smaller, the more G' deviates from G", and the more brittle (or glass-like) the material becomes.
Raman Spectroscopy
Raman spectroscopy measurements were performed using a Thermo Scientific iXR Raman spectrometer. The iXR system used a 532-nm laser operated at 10-mW power at the sample. Data acquisition and processing were carried out using the Thermo Scientific OMNIC for Dispersive Raman software. Sequential Raman spectra (in parallel with the rheological measurements) were collected over a predetermined time window using the time series collection function of the SERIES software within the OMNIC for Dispersive Raman package. Spectra were collected as an average of four 2-s sample exposures.
RheoRaman Coupling
The Raman spectrometer (Thermo Scientific iXR Raman spectrometer) and rheometer (Thermo Scientific HAAKE MARS 60) were coupled together using the Thermo Scientific HAAKE rheoRaman module. The iXR Raman spectrometer was free-space coupled to the rheometer with an optical train that used a series of mirrors to direct the incident laser into the rheoRaman module (Figure 1).
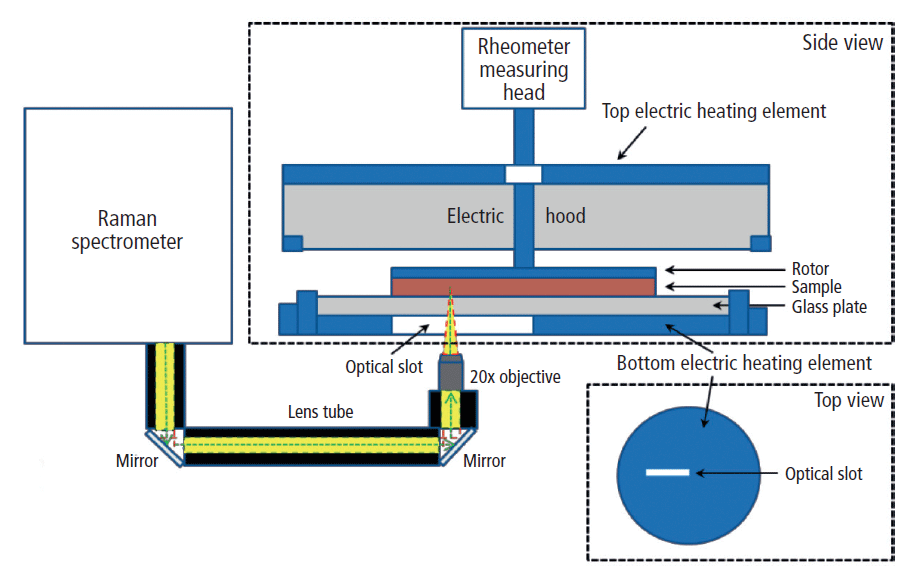
Figure 1: Schematic diagram of the rheoRaman system (MARSXR) showing side and top views of the rheometer sample stage. The iXR Raman spectrometer is free-space coupled to the MARS rheometer using lens tubes and mirrors that direct light into a 20x objective. The objective focuses the incoming laser (green dashed line) and collects the back-scattered Raman light (yellow) coming out of the sample sitting atop the rheometer stage.
The sample was positioned between a sandblasted glass bottom plate and the serrated 35-mm plate rotor to avoid slippage at the sample–plate interfaces. An electrical heating element within the rheoRaman module provided temperature control from below the sample, while an active electrical hood was used to provide temperature control from above (eliminating the potential for a temperature gradient within the sample). Cooling of the sample was supplied from a temperature-controlled water bath circulator.
Results and Discussion
Cocoa Butter Raman Spectroscopy
Full range (500–3100 cm-1) Raman spectra for the liquid-phase cocoa butter and the two investigated solid phase cocoa butter polymorphs (forms III and IV) are shown in Figure 2. Prominent Raman features were observed in both the C–H stretching region (2700–3050 cm-1) and the fingerprint region (1000–1800 cm-1). More specifically, the lower Raman shift features include the carbonyl (C=O) stretching region (1700–1800 cm-1), the olefinic (C=C) band at ~1655 cm-1, the CH3 and CH2 deformations (~1460 and 1440 cm-1, respectively), the CH2 twisting region (1250–1300 cm-1), and the C–C stretching region (1000–1200 cm-1).
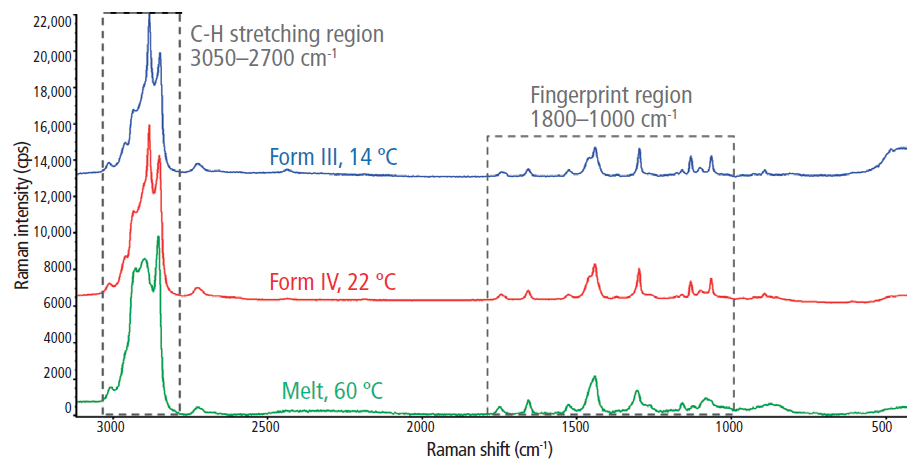
Figure 2: The full Raman spectra for the melt phase and two polymorphs (forms III and IV) of cocoa butter.
The C-H stretching regions for the melted and solidified cocoa butter specimens are highlighted in Figure 3. Two strong peaks were observed at ~2850 cm-1 and 2882 cm-1, which are attributed to symmetric and asymmetric CH2 stretching, respectively (2). The symmetric vibrational modes at 2850 cm-1 were dominant in the liquid (melt) phase, indicating reduced lateral interactions between CH2 groups and a low degree of intermolecular interactions. Conversely, the asymmetric vibrations at 2882 cm-1 were dominant in the solid phase, which suggests increased lateral interactions resulting from closely packed crystalline structures. Thus, the 2850 cm-1 and 2882 cm-1 bands are strong indicators of amorphous and crystalline content, respectively (8). Subsequently, the I2882/I2850 peak intensity ratio was used to dynamically track crystal formation during the in situ rheoRaman measurements.
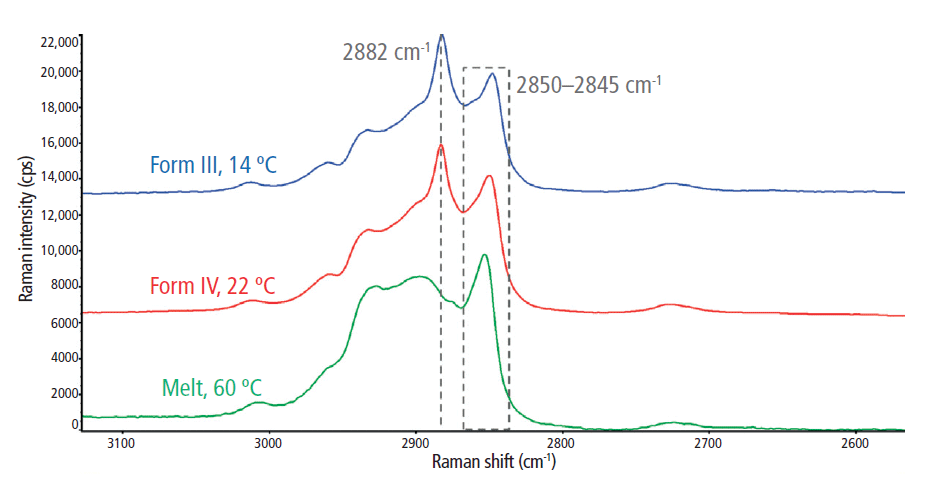
Figure 3: Raman spectra of the C-H stretching region (2700–3050 cm-1) for the melt phase and two polymorphs (forms III and IV) of cocoa butter.
Although they are less intense than the C-H stretching region, approximately eight unique spectral features were identified in the fingerprint region (1000–1800 cm-1; Figure 4). When comparing the cocoa butter melt state to the crystalline cocoa butter polymorphs, the most significant changes were observed in the C–C stretching region (1000–1200 cm-1). Two well-defined features emerged at 1130 cm-1 and 1063 cm-1 during the solidification process, which originate from the symmetric and asymmetric C-C stretching, respectively (9,10). In the melt phase, all C-C stretching bands were relatively weak and broad because of the disordering effects of methyl gauche conformations. However, as the cocoa butter solidified, the backbone methyl groups were ordered into the trans conformation, signified by the emergence of the peak at 1130 cm-1. Therefore, in addition to the I2882/I2850 peak intensity ratio, the I1130/I2850 spectral marker was also used to track the crystalline-phase transition within cocoa butter via in situ rheoRaman measurements.
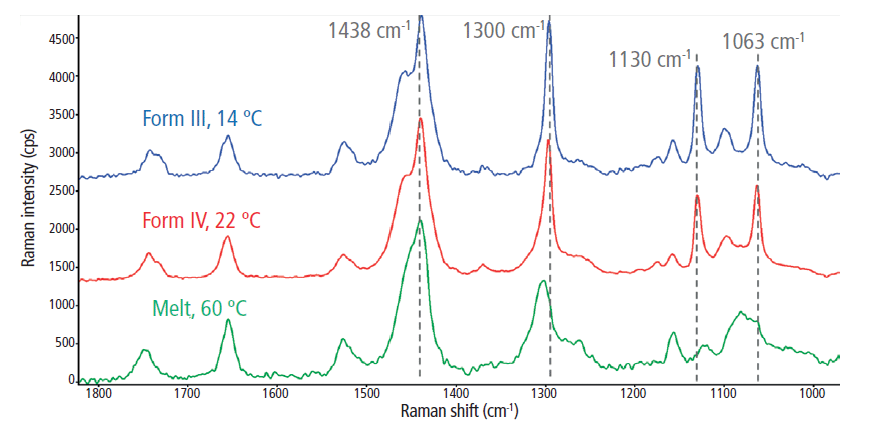
Figure 4: The 1000–1800 cm-1 Raman spectral range for the melt phase and two polymorphs (forms III and IV) of cocoa butter.
Form III: Simultaneous
Rheology and Raman Spectroscopy
To form cocoa butter polymorph form III, the temperature was ramped down from 60 °C to 14 °C at a rate of 2 °C/min. During cooling and before reaching the isothermal temperature of 14 °C, both the elastic and viscous moduli (G' and G", respectively) began to increase exponentially at ~20 min and ~20 °C (Figure 5a). From 20 to 25 min, as the temperature was further decreased and then held at 14 °C, G' and G" increased by approximately six and five orders of magnitude, respectively. The rapid increase in the moduli indicates the start of the solidification process, where the cocoa butter specimen transformed from a semisolid material into a firm solid. After the temperature reached the constant value of 14 °C, the elastic modulus began to plateau at 30 min, whereas the viscous modulus reached a maximum at ~25 min and then gradually decreased from ~25 to 55 min. From 55 min and beyond, both G' and G" remained constant. After 55 min, G' was about two orders of magnitude greater than G", suggesting that the cocoa butter was now a rigid, glass-like solid. From a purely rheological standpoint, the cocoa butter appeared to have fully crystallized.
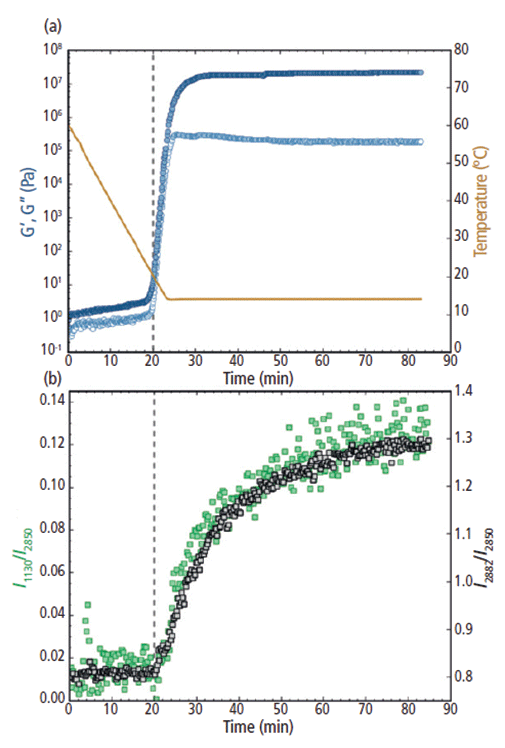
Figure 5: (a) Rheology: G' and G" (filled and open circles, respectively; plotted on the left y-axis) and the temperature trace (solid line; plotted on the right y-axis) and (b) Raman: the I1130/I2850 (left y-axis) and I2882/I2850 (right y-axis) intensity ratios for cocoa butter during the melt-crystalline transition to polymorph form III from 60 °C to 14 °C. The vertical dashed line at 20 min indicates the increase in both G' and G" and the Raman intensity ratios.
The rheological measurements for cocoa butter form III were further corroborated using in situ Raman spectroscopy (Figure 5b). Similarly to G' and G", both the I1130/I2850 and I2882/I2850 peak intensity ratios remained relatively constant during the first ~20 min of the measurement. Then the I1130/I2850 and I2882/I2850 spectral markers began to abruptly increase at ~20 min, indicating the formation of crystalline structures within the cocoa butter. As the cocoa butter further crystallized, both spectral ratios continued to increase from 20 to 70 min. After ~70 min, the Raman peak intensity ratios began to stabilize as the cocoa butter TAGs arranged into their final crystal structure. Significant scatter in the y-direction was observed for the I1130/I2850 ratio, especially at the earlier melt state and at the latter part of the crystallization process. This scatter was most likely because of the low intensity of the 1130 cm-1 feature in comparison to the strong Raman bands in the C-H stretching region (2850 cm-1 and 2882 cm-1).
Notably, the initial increase in G' and G" directly aligned with the rise of the 1130 and 2882 cm-1 spectral ratios. The concurrent increases in both the rheological response and Raman feature intensity suggests that the bulk solidification during the formation of cocoa butter polymorph form III was directly associated with the formation of crystalline structures. Interestingly, as the change in rheological behavior began to subside at 55 min, the Raman spectral features were still increasing and they did not become steady until ~70 min and beyond. It is postulated that although the overall bulk hardening of the cocoa butter had stopped, the crystalline domains were still rearranging at the molecular level and that the cocoa butter had not reached its final crystalline lattice structure until the end of the measurement.
Form IV: Simultaneous
Rheology and Raman Spectroscopy
To form cocoa butter polymorph form IV, the temperature was rapidly decreased from 60 °C to 22 °C at a rate of 10 °C/min. Note that since the cooling step was so brief, only the data from the isothermal step is shown in Figure 6. During the initial portion of the isothermal hold from 0 to 5 min (immediately following the rapid decrease in temperature from 60 °C to 22 °C), both G' and G" increased as the cocoa butter transformed from a melted liquid to a soft semisolid (Figure 6a). This initial increase in modulus is most likely because of a delay between the set temperature and the internal temperature of the loaded sample. After the sample had reached thermal equilibrium and was at the isothermal set point of 22 °C, the moduli were relatively stable from 10 to 25 min. From 25 to 50 min, however, both G' and G" began to gradually increase and then from 50 to 80 min, the moduli rapidly increased, where G' and G" increased by approximately five and four orders of magnitude, respectively. The exponential increase in the moduli indicates a solidification process, where the cocoa butter transformed from a pliable semisolid to a more robust, hardened solid. At 80 min and beyond, growth in the elastic modulus slowed and eventually plateaued, showing no further significant change past 100 min. The viscous modulus, however, reached a slight plateau from 80 to 100 min but continued to decrease from 100 min and beyond.
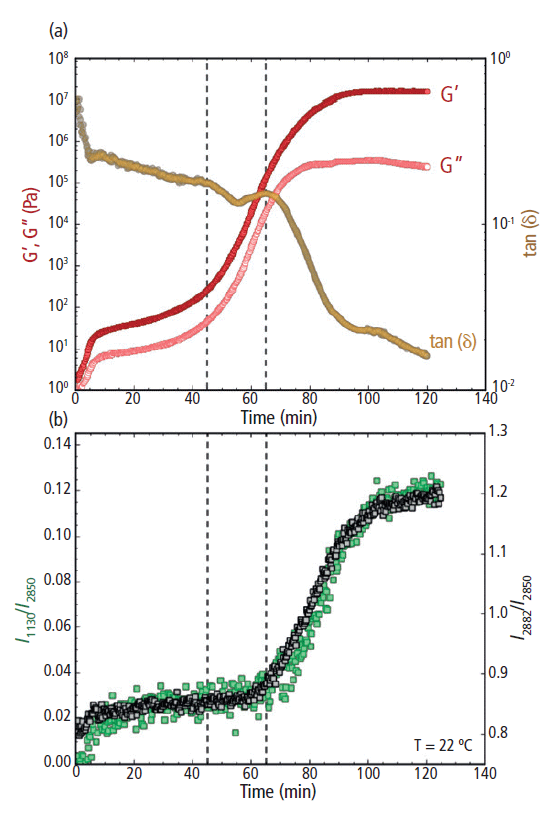
Figure 6: (a) Rheology: G' and G" (filled and open circles, respectively; plotted on the left y-axis) and tan(δ) (plotted on the right y-axis) and (b) Raman: the I1130/I2850 (left y-axis) and I2882/I2850 (right y-axis) peak intensity ratios for cocoa butter during isothermal crystallization at 22 °C. The vertical dashed line at 45 min indicates the increase of G' and G", while the dashed line at 65 min indicates the decrease in tan(δ) and increase in the Raman intensity ratios.
During the increase in G' and G", a rapid decrease in the loss factor tan(δ) was observed from ~65 min and beyond (Figure 6a, right y-axis). The decrease in the loss factor indicates a deviation in overall magnitude between G' and G". As the cocoa butter hardened, the increase in G' exceeded the increase in G", triggering the decrease in tan(δ). At the end of the 120 min isothermal study, G' was more than a full order of magnitude greater than G" and the loss factor was approaching 0.01, indicating the cocoa butter had transitioned into a brittle glass-like solid.
The observed rheological behavior was further confirmed using simultaneous Raman spectroscopy (Figure 6b). Initially, both the I1130/I2850 and I2882/I2850 peak intensity ratios remained unchanged during the first ~65 min of the isothermal study. Then a sharp increase of the I1130/I2850 and I2882/I2850 ratios began at ~65 min, indicating the formation of crystal structures within the cocoa butter. As the cocoa butter further crystallized, both spectral markers continued to increase from 65 to 100 min. Beyond 100 min, the growth in both Raman features had subsided and the peak intensity ratios began to stabilize.
Overall, the rate of increase in the 1130 and 2882 cm-1 peak intensity ratios were similar to the rate of change for both G' and G" during the formation of cocoa butter polymorph form IV (Figures 6a and 6b). However, there was a noticeable 15–20 min lag between the observed increase in G' and G" and the rise of the Raman intensity ratios. The sharp upturn in G' and G" indicates an increased resistance to deformation (that is, a bulk hardening of the cocoa butter), signaling the start of the solidification process. The Raman spectral markers, on the other hand, are indicators of crystal formation. Thus, the time delay between the rheology and Raman profiles suggests that cocoa butter first hardens into an amorphous solid, followed by a transformation from an amorphous to a crystalline solid. This morphological transformation was signified by the subsequent increase in the Raman band intensities associated with crystal cocoa butter structures (the 1130 and 2882 cm-1 peaks). The temporal separation of the rheological and Raman spectral profiles indicates a clear distinction between bulk hardening of the cocoa butter and the formation of crystalline domains during the formation of polymorph form IV.
Interestingly, the increase in the Raman spectral features (I1130/I2850 and I2882/I2850) directly correlated with the observed reduction in tan(δ) (Figures 6a and 6b). The loss factor is an indication of material brittleness and crystalline structures are commonly known to be brittle. Thus, it is reasonable to infer that the formation of crystal domains at the molecular level (as indicated by Raman) coincides with the overall brittleness of the cocoa butter. As a result, the loss factor may be a more revealing indicator of bulk cocoa butter crystallization than G' and G" alone.
Conclusions
Simultaneous rheology and Raman spectroscopy measurements were used to examine the isothermal crystallization of cocoa butter. This multimodal analytical technique allowed the bulk mechanical properties of cocoa butter (G', G", and tan[δ]) to be directly correlated with conformational changes at the molecular level (νas[CH2] mode at 2882 cm-1 and the νs[C-C] mode at 1130 cm-1) in real-time. With a slow cooling (2 °C /min) and subsequent isothermal hold at 14 °C, the concurrent increases in both the rheological response and Raman feature intensity suggests that the bulk solidification during the formation of cocoa butter polymorph form III coincided with the formation of crystalline structures. With a rapid cooling (10 °C/min) followed by an isothermal hold at 22 °C, however, there was a noticeable time lag between the rheological response (G' and G") and the Raman spectral profiles during the formation of cocoa butter polymorph form IV. The observed time delay indicates that cocoa butter crystallized by first hardening into an amorphous solid, manifested by a sharp increase in G' and G" while the Raman features remained unchanged. The amorphous solid then underwent a morphological transition to form a crystalline solid, signified by the increase in Raman features associated with crystal cocoa butter structures (1130 and 2882 cm-1). Without coupling these two separate analytical techniques, the observed amorphous-solid to crystalline-solid transformation would have been left undetected. Alone, each technique suggests a single stage process, however, only when the two techniques are coupled is the multiphase crystallization process revealed, further exemplifying the unique analytical capability unleashed by hyphenating rheology with in situ Raman spectroscopy.
References
(1) K. Sato, Crystallization of Lipids: Fundamentals and Applications in Food, Cosmetics, and Pharmaceuticals (John Wiley & Sons, Hoboken, New Jersey, 2018).
(2) S. Bresson, D. Rousseau, S. Ghosh, M. El Marssi, and V. Faivre, Eur. J. Lipid Sci. Technol. 113, 992–1004 (2011).
(3) R. Wille and E. Lutton, J. Am. Oil Chem. Soc. 43, 491–496 (1966).
(4) K. Dewettinck, I. Foubert, M. Basiura, and B. Goderis, Cryst. Growth Des. 4, 1295–1302 (2004).
(5) C. Loisel, G. Keller, G. Lecq, C. Bourgaux, and M. Ollivon, J. Amer. Oil Chem. Soc. 75, 425–439 (1998).
(6) A.G. Marangoni and S.E. McGauley, Cryst. Growth Des. . 3, 95–108 (2002).
(7) K. van Malssen, A. Langevelde, R. Peschar, and H. Schenk, J. Amer. Oil Chem. Soc. 76, 669–676 (1999).
(8) R.G. Snyder, H.L. Strauss, and C.A. Elliger, J. Phys. Chem. 86, 5145–5150 (1982).
(9) R.J. Meier, Polymer 43, 517–522 (2002).
(10) M. Zheng and M. Du, Vib. Spectrosc. 40, 219–224 (2006).
Nathan C. Crawford and Rui Chen are with Thermo Fisher Scientific in Madison, Wisconsin. Mohammed Ibrahim is with Thermo Fisher Scientific in San Jose, California. Direct correspondence to: nathan.crawford@thermofisher.com
