Improving ICP-MS Analysis of Samples Containing High Levels of Total Dissolved Solids
A novel approach to handling samples with very high and variable levels of sample matrix is to use aerosol dilution, whereby the amount of sample aerosol that reaches the plasma is reduced by dilution with an additional argon gas stream.
A novel approach to handling samples with very high and variable levels of sample matrix is to use aerosol dilution, whereby the amount of sample aerosol that reaches the plasma is reduced by dilution with an additional argon gas stream. This allows the inductively coupled plasma–mass spectrometry (ICP-MS) system to directly measure samples containing matrix levels of up to 25% total dissolved solids (TDS), which is more than a factor of 100 higher than the accepted maximum. Samples containing variable levels of NaCl were measured against simple aqueous standards, and accurate spike recoveries were obtained for all levels of matrix without requiring any prior knowledge of matrix levels, and without predilution of the samples.
Since inductively coupled plasma–mass spectrometry (ICP-MS) became commercially available in the 1980s, the technique has been adopted for trace-element analysis across a wide range of industries and applications. One of the barriers to wider acceptance of the technique has been its limited tolerance to the high matrix levels found in many samples encountered in environmental, clinical, food, nuclear, and geological applications. In contrast to ICP–optical emission spectrometry (ICP-OES), ICP-MS uses an interface to transfer ions into a high vacuum region for separation and detection. This interface includes plates or "cones" with small-diameter orifices to allow ions to pass through while the vacuum is maintained. When samples that contain high levels of total dissolved solids (TDS) are analyzed, the dissolved matrix can deposit on the interface cone orifices, leading to signal loss and instability.
When the sample contains a high concentration of easily ionized elements such as Na and K, the signal for analyte elements can also be reduced because of ionization suppression. This effect occurs when the plasma is flooded with free electrons from the easily ionized matrix elements, which reduces the ionization of analyte elements. Ionization suppression particularly affects those analytes with higher ionization potentials such as the commonly measured trace elements As, Se, Cd, and Hg (1). A very high concentration of a matrix element may also cause defocusing of the extracted ion beam because of space charge effects, causing further significant loss of analyte sensitivity (2).
A high level of matrix also changes a sample's physical parameters such as viscosity and surface tension, which affects the uptake and nebulization of the liquid; these effects can usually be corrected by using internal standardization.
A further problem encountered in ICP-MS is that of spectral interferences, where high levels of matrix elements such as Na, S, Cl, K, and Ca give rise to polyatomic ions that overlap the major isotopes of Ti, V, Cr, Ni, Cu, Zn, As, and other elements in the mass spectrum (3). For these reasons, as well as lower capital cost and higher sample throughput, ICP-OES is often used for the analysis of high salt samples (4,5), but ICP-OES has reporting limits typically in the microgram-per-liter range, rather than the nanogram-per-liter range offered by ICP-MS.
The limited matrix tolerance of the ICP-MS interface is the main reason for the commonly accepted maximum recommended limit of 0.2% (2000 ppm) TDS in samples intended for ICP-MS analysis. The 0.2% TDS (or 2 g/L) limit is also defined in many standard methods for ICP-MS, such as EN-ISO 17294-2 ("Water Quality") (6), US-EPA 6020 ("Water and Wastes") (7), and EN 13805 ("Trace Elements in Food") (8). For many high matrix samples or sample digests, this TDS limit means that a dilution step is required before ICP-MS analysis. Conventional off-line sample dilution adds time and cost to the analysis, introduces the risk of contamination and dilution errors, and increases the volume of waste that must be disposed of. Automated on-line sample dilution simplifies the process, but the extra pumps and valves are often expensive and the additional tubing and connections are prone to leaks and blockages; on-line autodilution also carries the risk of contamination from the diluent.
Alternative approaches to measuring high salt samples by ICP-MS include matrix removal, using a technique such as on-line chelation (9–12), solid-phase extraction (13), or co-precipitation (14,15). These techniques can be applied to the routine analysis of certain elements, but they are time-consuming and require a skilled operator. Moreover, because of their similar chemical properties, some elements such as Br, Rb, I, and Cs may be removed along with the matrix elements.
Another option to reduce the exposure of the ICP-MS interface to high salt levels is to introduce only a small volume of sample solution, using a discrete sampling or flow injection approach (16,17). This technique injects a small volume of sample into a continuous carrier stream, so it reduces the total amount of matrix that reaches the plasma per sample, and thereby increases the number of samples that can be measured before interface maintenance is required. However, discrete sampling does not address the issues of ionization suppression and space charge effects because the undiluted sample matrix is aspirated during the short period of the measurement. The short duration of the signal from the discrete sample volume also limits the flexibility of the acquisition method, because it may impose shorter-than-optimum integration times or limit the number of different acquisition or cell gas modes that can be run.
However, there is a need for low-level trace-element analysis of high matrix samples, so improving the matrix tolerance of ICP-MS is a primary goal for instrument manufacturers. In addition to environmental monitoring of high salt matrix samples such as open ocean and coastal seawater (18), enclosed lakes and lagoons with salt levels far in excess of oceanic seawater are also of interest. Known as hypersaline lakes, these bodies of water include Don Juan Pond and Lake Vanda in Antarctica, Lake Assal in Djibouti, Garabogazköl in Turkmenistan, the Dead Sea between Israel and Jordan, and Mono Lake and the Great Salt Lake in the United States. These hypersaline lakes may contain salt levels many times higher than seawater, with more than 40% salinity in the case of Don Juan Pond.
Other high salt matrix samples such as saturated salt water (25% NaCl) used for dialysis, fish transportation, and during the development of oil fields may also require analysis.
A new approach to high matrix analysis by ICP-MS is the use of aerosol dilution (19), which eliminates manual sample handling while retaining the standard ICP-MS sample introduction hardware and operating conditions, apart from the use of a lower nebulizer gas flow rate. The peristaltic pump delivers sample solution to the nebulizer at a normal uptake rate of ~0.25 mL/min, but the reduced nebulizer gas flow means that the volume of sample aerosol created decreases. A diluent gas flow (of argon) is then added between the ICP-MS spray chamber and torch to dilute the aerosol as it passes to the plasma. Aerosol dilution reduces the amount of sample aerosol that reaches the plasma, effectively diluting the sample matrix without requiring any additional hardware, addition of reagent or diluent solutions, or manual sample handling. Aerosol dilution reduces the total mass of sample aerosol that reaches the plasma, so plasma loading is reduced and more energy remains for matrix decomposition and analyte ionization. This means that both ionization suppression and matrix deposition on the interface cones are reduced. While aerosol dilution reduces sensitivity the same way as liquid dilution, it has the benefit of eliminating the risk of dilution errors and contamination, and it is simpler and more reliable than online liquid dilution.
This article describes the use of a new aerosol dilution approach to improve the matrix tolerance of an ICP-MS instrument, allowing samples with matrix levels of up to 25% salt to be run routinely. The capability to measure such high matrix levels by ICP-MS is unprecedented and removes a long-standing limitation of the technique. In combination with a collision–reaction cell (CRC) operating in helium (He) mode for the removal of matrix-based polyatomic ion overlaps (20), aerosol dilution ICP-MS offers a complete solution for accurate trace-element analysis of high matrix samples.
Experimental
Instrumentation
An Agilent 7900 ICP-MS system fitted with standard nickel sampling and skimmer cones, a standard glass nebulizer, quartz spray chamber chilled at 2 °C, and a quartz torch with a 2.5-mm injector was used. The Ultra High Matrix Introduction (UHMI) option was fitted and the argon carrier gas was humidified to reduce salt build up at the nebulizer. The ICP-MS system includes a fourth generation CRC, the ORS4, operating in He collision mode. The optional H2 cell gas line was fitted and H2 cell gas was used for improved interference removal on elements with intense plasma-based polyatomic overlaps (Ca [m/z 40] Fe [m/z 56], and Se [m/z 78]). An Agilent ASX-520 autosampler was used for sample delivery.
Calibration Standards and Sample Preparation
Several salt matrix solutions containing levels of NaCl up to 25% were prepared by dissolving reagent-grade NaCl (Merck EMSURE) in deionized water stabilized with the addition of 0.5% HNO3 and 0.6% HCl. Calibration standards were prepared from custom mix and single-element stock solutions purchased from Inorganic Ventures, and spike mixes were prepared from mixed quality control (QC) stock solutions (CPI International) and a single-element Hg standard (Merck). A mixed internal standard solution was also prepared from single element stocks from the same suppliers and added on-line using the internal standard mixing tee-connector.
Analytical Method
UHMI 100 (100× aerosol dilution) was selected for maximum robustness and matrix tolerance, although NaCl is more easily decomposed than some refractory matrices such as CaCO3 and SiO2, and can be run successfully at a lower aerosol dilution factor. After setting the aerosol dilution factor, the other instrument settings were optimized using the autotune function run automatically during the instrument startup sequence. Tune settings for both tune modes (He mode for most elements and H2 mode for Ca, Mn, Fe, and Se) were optimized independently, but most parameters are consistent for the two gas modes. The ICP-MS instrument settings used are shown in Table I.
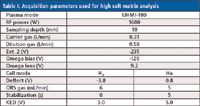
Table I: Acquisition parameters used for high salt matrix analysis
A range of trace elements of interest in environmental samples was measured in salt matrices ranging from zero added NaCl to 25% NaCl solution. All samples were measured against simple aqueous calibration standards (no NaCl matrix), prepared in the same acid mix as the samples (0.5% HNO3 and 0.6% HCl). Internal standards were added on-line to correct for physical sample transport effects and any residual matrix suppression.
Results
The matrix level will affect the sample transport and nebulization processes even when aerosol dilution is used, so overall sensitivity may be reduced in very high matrix samples. But these physical processes affect the total amount of aerosol that is produced and transported to the plasma, so changes should be consistent for all elements. This can be observed in the plot of relative intensities for the internal standards measured in the variable matrix samples containing between 0% NaCl and 25% NaCl, shown in Figure 1. The internal standards included easily ionized elements (6Li, Sc, Rh, In) and poorly ionized elements (Ge, Ir). The sensitivity of all the internal standards was reduced by about the same amount, indicating that the signal reduction was mostly because of physical transport effects. If the sensitivity reduction was mainly because of ionization suppression from the NaCl matrix, the poorly ionized elements would have been affected much more than the easily ionized elements.

Figure 1: Relative internal standard intensities measured in variable NaCl matrix solutions.
To demonstrate the effectiveness of the aerosol dilution method to extend the matrix tolerance of ICP-MS, spike recoveries for a range of elements were measured in the series of different NaCl matrices. The spike recovery data are shown in Figure 2 for the "big four" toxic trace elements and Figure 3 for four elements that suffer severe overlap from NaCl-based polyatomic interferences. Some of these polyatomic interferences are well documented in ICP-MS such as ArCl+ on As+ at m/z 75 (Figure 2), and ClO+ on V+ at m/z 51 (Figure 3). Others such as NaCl+ on Ni+ at m/z 60, and ArNa+ on Cu+ at m/z 63 (Figure 3) are not as well known because they only become a significant issue when very high levels of NaCl matrix are measured. However, the consistent spike recoveries demonstrate that He mode is effective at reducing all of these matrix-based polyatomic interferences.
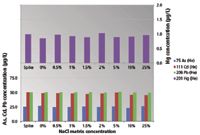
Figure 2: Spike recoveries for As, Cd, Hg, and Pb in variable NaCl matrices with up to 25% NaCl. The spike level added is shown as the first data point.
Many of the elements measured (As, Cd, Hg) are relatively poorly ionized and would be expected to suffer severe signal loss from ionization suppression in a matrix containing such high levels of an easily ionized element. The consistent spike recoveries achieved in all levels of NaCl matrix demonstrate that aerosol dilution is effective in minimizing the impact of ionization suppression in variable samples containing salt concentrations up to 25% NaCl, a level that would not previously have been considered suitable for analysis by ICP-MS.
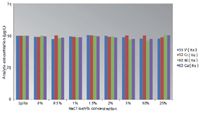
Figure 3: Spike recoveries for interfered elements V, Cr, Ni, and Cu in variable NaCl matrices with up to 25% NaCl. The spike level added is shown as the first data point.
The NaCl matrix was measured repeatedly to check the long term matrix tolerance of the method. Solutions containing 25% NaCl were prepared with and without a multielement QC spike (50 μg/L for most elements except As: 25 μg/L). The spiked and unspiked samples were measured alternately for a period of more than 4 h. Spike concentrations were calibrated against the nonmatrix matched (no NaCl) calibration. The results are presented in Table II, demonstrating accurate recovery within ±10% of the spike level, and precision well below 5% relative standard deviation (RSD) for most elements measured.
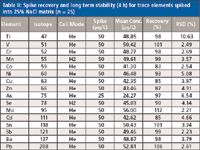
Table II: Spike recovery and long term stability (4 h) for trace elements spiked into 25% NaCl matrix (n = 25)
Conclusions
An ICP-MS system with aerosol dilution was used for the direct analysis of trace elements in salt matrix samples up to 25% NaCl, without prior sample dilution.
Accurate recoveries and excellent long term stability were obtained for microgram-per-liter level spikes for all analytes. The elements of interest included several that suffer from severe polyatomic overlaps in the NaCl matrix, so the consistent recoveries demonstrate that polyatomic interferences were reduced effectively using He mode in the collision–reaction cell.
Ed McCurdy is an ICP-MS product manager at Agilent Technologies LDA (UK) Ltd., in Cheshire, UK. Wim Proper is a development analyst with Eurofins Analytico BV in Barneveld, Netherlands. Direct correspondence to: ed_mccurdy@agilent.com
References
(1) D. Beauchemin, J. W. McLaren, and S. S. Berman, Spectrochim. Acta Part B 42(3), 467–490 (1987).
(2) I. Rodushkin, T. Ruth, and D. Klockare, J. Anal. At. Spectrom. 13(3), 159–166 (1998).
(3) N.M. Reed, R.O. Cairns, and R.C. Hutton, J. Anal. At. Spectrom. 9, 881–896 (1994).
(4) P.F. Kehr, J.S. Jones, D.A. Fritz, D.E. Harrington, and W.R. Bramstedt, At. Spectrosc. 6(5), 128–133 (1985).
(5) W. Li, F. Pan, S. You, Q. He, D. Kang, and Y. Xu, Spectrochim. Acta Part B 42(6), 853–858 (1987).
(6) International Organization for Standardization (ISO) 17294-2:2003 Water quality — Application of Inductively Coupled Plasma Mass Spectrometry (ICP-MS) - Part 2: Determination of 62 Elements (Geneva, Switzerland, 2009).
(7) United States Environmental Protection Agency, Method 6020A Inductively Coupled Plasma-Mass Spectrometry. Retrieved from http://www.epa.gov/wastes/hazard/testmethods/sw846 (United States Environmental Protection Agency, 2007).
(8) German Institute for Standardization, DIN EN 13805:2002 Foodstuffs — Determination of Trace Elements - Pressure Digestion (German Institute for Standardization, Berlin, Germany).
(9) L. Ebdon, A. Fisher, H. Handley, and P. Jones, J. Anal. At. Spectrom. 8(7), 979–981 (1993).
(10) R.E. Sturgeon, S.S. Berman, A. Desaulniers, and D.S. Russell, Talanta 27, 85–94 (1980).
(11) F.A.M Silva, C.L.P. Da Silveira, N. Miekekey, and I.L. Kuechler, Anal. Sci. 20(9), 1295–1299 (2004).
(12) G.M. Greenway, S.M. Nelms, and D. Koller, Anal. Commun. 33, 57–59 (1996).
(13) T.-t. Shih, W.-y. Chen, and Y.-c. Sun, J. Chromatogr. A 1218(16), 2342–2348 (2011).
(14) A.S. Buchanan and P. Hannaker, Anal. Chem. 56(8), 1379–1382 (1984).
(15) S. Kagaya, S. Miwa, and K. Tohda, Anal. Sci. 23(8), 1021–1024 (2007).
(16) M.J. Bloxham, S.J. Hill, and P.J. Worsfold, J. Anal. At. Spectrom. 9, 935–938 (1994).
(17) S.N. Willie, Y. Iida, and J.W. Mclaren, J. Anal. At. Spectrom. 19, 67–72 (1998).
(18) J. McLaren, D. Beauchemin, and T.V. Voet, Can. J. Spectrosc. 30(6), 29A–32A (1985).
(19) S.M. Wilbur and L.C. Jones, The Open Chemical and Biomedical Methods Journal 3, 135–142 (2010).
(20) E. McCurdy and G. Woods, J. Anal. At. Spectrom. 19, 607–615 (2004).

Inside the Laboratory – The Petrochronology Group at University of California, Santa Barbara
January 29th 2024In this edition of “Inside the Laboratory,” John Cottle, PhD, a professor of geology at the University of California, Santa Barbara, and a member of Spectroscopy’s Editorial Advisory Board, discusses his group’s most recent work using “laser ablation split steam” analysis to measure elemental concentrations and isotopic ratios in rocks and minerals.