Ion Mobility Spectrometers as Chromatographic Detectors
Special Issues
Interest in connecting ion mobility spectrometry (IMS) to GC and especially to LC is now growing. One favorable property of IMS is that it can work with ambient pressure and can be easily connected to a gas or liquid chromatograph. Analytical applications of GC–MS and LC–MS are very different and encompass investigations into food, medical science, environment, drugs of abuse, chemical warfare agents, and explosives.
Chromatography connected with ion mobility spectrometry (IMS) is not commonly used, but is being investigated more. IMS is an independent analytical technique with very good detectability and a rather small separation ability. One favorable property of IMS is that it can work with ambient pressure and can be easily connected to a gas chromatograph. Analytical applications of gas chromatography coupled to ion mobility spectrometry (GC–IMS) are very different and encompass investigations into food, medical science, environment, drugs of abuse, chemical warfare agents, and explosives.
Chromatography is the most important method of organic compound analysis, and gas chromatographs are found in analytical laboratories worldwide. Gas chromatography (GC) can analyze approximately 20% of over 112 million chemical compounds registered in the Chemical Abstracts Service (www.cas.org). Almost all other chemical substances may be analyzed using liquid chromatography (LC). These chemicals are tested with the use of stationary devices or portable apparatus suitable for various environmental conditions.
Hybrid instruments, such as chromatographs coupled with mass spectrometers, are now widely used. The disadvantages of gas or liquid chromatography–mass spectrometry (GC–MS or LC–MS) devices include their price, high cost of operation, complex construction, and the need for a high vacuum. Therefore, for the analysis of small amounts of chemical compounds contained in mixtures, chromatographs combined with ion mobility spectrometers are being increasingly used. Such instruments allow results to be obtained quickly and at a low cost. In 2008, a paper on applying ion mobility spectrometers as GC detectors was published (1). In this review article we describe the results of studies connecting chromatography to ion mobility spectrometry (IMS) that have been published since this important paper.
Ion Mobility Spectrometry
In the 1970s, IMS, initially called plasma chromatography, was invented (2). The rapid development of this analytical technique, now called IMS, took place in the 1980s (3). The core of IMS is that the analyte molecules are converted into ions, which move within the electric field in the gas (usually air) at atmospheric pressure and at a relatively low temperature.
Ionization of the analyte molecules is predominantly the effect of ion-molecule reactions. β radiation emitted by 63Ni, or less commonly 3H, is used for the production of primary ions. Some components of the gas flowing through the spectrometer (for example, water vapor and carbon dioxide) interact with the primary ions to form reactant ions. These ions react with the analyte molecules to create positive or negative ionic products. The velocity of their movement in the gas is dependent on the value of the electric field and on the properties of the ions and the drift gas molecules. Ions are identified by the values of mobility coefficient, that is, the ratio of the ion velocity to the value of the electric field.
There are currently several types of IMS, which differ in the way that they identify ions. The most important among them are classic IMS, where detectors with a drift tube are used (DT IMS) (4) and differential mobility spectrometry (DMS) (5–7). The scheme of classical IMS is shown in Figure 1a. It consists of two sections: the reaction region and the drift region. In the reactant region, analytes are ionized. Portions of ions from this section are introduced into the drift section through the ion grid constituting the electric valve controlled by voltage pulses (8). In the drift region, ions are separated by the differences in the drift time resulting from their different mobilities (3).
Figure 1: The two most popular constructions of ion mobility spectrometers: (a) with drift tube and (b) differential mobility spectrometer.
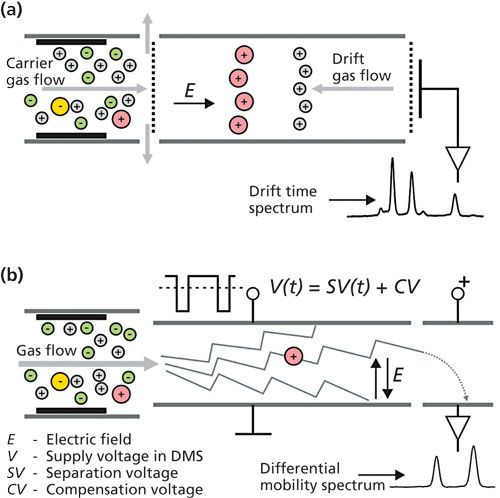
Differential mobility spectrometers are constructed in plane (Figure 1b) or cylindrical geometry (3,6,9). In differential ion mobility spectrometers, a high-intensity alternating electric field with a direction perpendicular to the gas flow is used. The separation of ions is based on a mobility coefficient, which depends on the electric field. The immediate result of the DMS measurement is compensating voltage spectrum (7). DMS can be easily miniaturized (10,11).
Ion mobility spectrometers of both types are characterized by very good detectability but low resolving power. Therefore, they are coupled to chromatographic columns.
Connecting Ion Mobility Spectrometers to Gas Chromatographs
The first gas chromatograph to be coupled with an ion mobility spectrometer was described by Karasek and Keller (12). Combining chromatography with IMS is easier than combining it with MS. However, it is necessary to take into account the fact that the flow of the carrier gas through the capillary column is much smaller than the flow of the carrier gas through the spectrometer. Moreover, the time constant of the spectrometer should be chosen according to the column dynamic parameters. In chromatographs coupled to IMS, capillary columns (CCs) and multicapillary columns (MCCs) are used (1,13–19). Capillary columns have dimensions similar to the columns used in regular chromatographs, with a length of tens of meters. It can be a capillary with a diameter of 0.53 mm and a stationary phase thickness of 0.25 mm in the form of a coil with a diameter of 50 mm and a length of 6 m. In contrast, MCCs are short columns-they can be 17 cm (18), 20 cm (13,14), or 25 cm (17) in length. As well as the usual columns, resistively heated columns are also used (20).
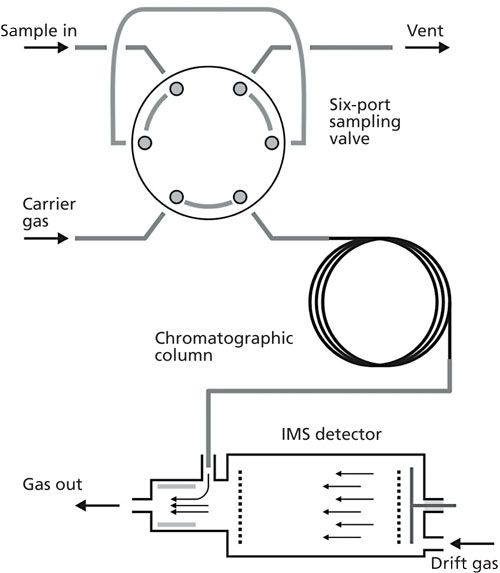
The chromatograph can be connected to the IMS via the transfer line but often the column outlet is placed axial or side directly in the reaction section of the spectrometer (19). Popular IMS spectrometers used as detectors work in so-called “unidirectional flow” systems in which drift gas flows not only through drift region but also through the ionic reactor. In such devices the sample is introduced through the input connection placed in the vicinity of the ionization source. The volume of the region in which the sample is present in the detector is therefore very small. It means that the time constant of the detector is low, but, on the other hand, the efficiency of sample ionization is also not high. The scheme of the GC–IMS system with a sampling device based on a six-port valve is shown in Figure 2.
Figure 2: Connection of ion mobility spectrometer with unidirectional flow to chromatographic column and typical sampling system.
Ion mobility spectrometers that can operate independently are commonly coupled with chromatographs in research laboratories (21–23). Precoupled GC–IMS devices are also commercially available. GC–IMS can be easily miniaturized (1,15,20,24,25) and even chip designs can be used for this purpose. As an example, a column 3-m long and 150-µm wide, 240-µm deep etched in a 3.2-cm2 silicon chip and miniaturized DMS has been developed (26). Small, light GC–IMS devices devoid of moving parts are also resistant to changing operation conditions and consume little power, thus they fulfill the requirements of portable instruments (1,15,20,27,28). By definition, ion mobility spectrometers are instruments that perform very fast analysis. Therefore, it is advantageous to combine them with the chromatographs operating fast GC (14,20,29). Analysis by GC–IMS is usually completed within 3 to 5 min.
The components of the mixture separated in the column are introduced into the reaction region of the ion mobility spectrometer where they are ionized. They then enter the second part of the spectrometer in the form of positive or negative ions, where they move in the electric field with different velocities depending on their mobility. The mobility of the ions depends on their charge and shape and so it may differ between isomers. The mobility of ions affects their drift time through the spectrometer. This time at the same conditions is a characteristic value and allows identification of the ions. When using the DT–IMS technique two modes of detection are possible-selective and non-selective. In nonselective mode, the detector signal is reactant ion charge. A reduction in the charge can be caused by the presence of various analytes. In selective mode, the peak corresponding to the ions of a specific mobility is recorded. The intensity of the peak is dependent on the presence and concentration of selected analytes.
When DMS is used as the chromatographic detector, a two-dimensional (2D) signal is most commonly recorded-that is, the dependence of the ionic current on the retention time and the compensation voltage. The measurement of the full dispersion plots (dependency of ionic current on separation and compensation voltage) requires a long time and therefore is not used in GC–DMS systems.
The IMS spectra are dependent on the concentration of the analytes, and their shape can be different for a substance in various concentrations. This is related to the formation of dimer ions. For example, at low concentrations at atmospheric pressure dimers are rarely formed. The presence or absence of dimers containing the same substance can lead to false identification of analytes (3).
Quantitative analysis with IMS is difficult because of the narrow linear dynamic range of spectrometers. Good results of quantitative analysis are obtained with small quantities of analytes. The linear dynamic range of measurement with IMS is from 1 ppb to 1 ppm, depending on the type of analyte (30).
Quantitative GC–IMS data is nonlinear in high concentrations. This is related to the use of 63Ni in the ionization of chemical compounds that are eluted from the chromatographic column.
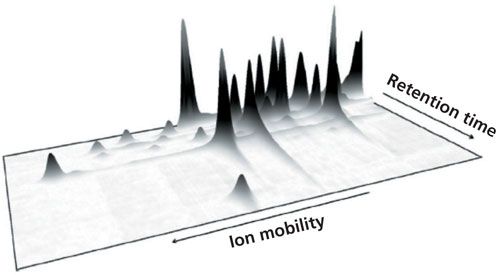
With the GC–IMS, 2D information can be obtained because signal intensity is a function of retention and drift time (23). An example of such dependency is shown in Figure 3. When IMS is combined with 2D gas chromatography (GCxGC), three-dimensional (3D) analytical information is obtained. When processing multidimensional analytical information, special algorithms allowing the identification and determination of analyte concentration are applied (13,21,24,28).
Figure 3: An example of 2D measurement results obtained with GC–IMS system. Adapted and reproduced with permission from reference 35.
Identification of ions with GC–IMS, which is not as good as with GC–MS, can be verified, especially during the development of analytical procedures, using MS (31). For analyte identification, the use of a corona discharge ion mobility spectrometer coupled to GC can also yield good results (15). The use of an ion mobility spectrometer as a chromatographic detector for monitoring positive ions may produce signals similar to those generated by a flame ionization detector (FID). A 2D chromatogram, similar to that produced with the FID, is obtained. It has been demonstrated that much smaller amounts of ketones can be detected when using GC–IMS rather than GC–FID (15). Detectability using GC–IMS can be further improved by reducing the noise. An algorithm that yielded significant signal-to-noise enhancement by averaging and smoothing the denoised signals was developed by Ghorashi and colleagues. (29). This is particularly important for the quantitative analysis.
Applications of GC–IMS Systems
Analysis with GC–IMS consists of five stages: sample introduction, component separation in the column, component ion production, ion separation, and recording of drift time or compensation voltage values. It is possible to distinguish the chemical compounds with the same molecular weight but a different structure. Under field conditions, the simplest way is to introduce air or an aerosol to the GC system through the open sample inlet, with the possibility of pyrolyzing the analytes. The detection of analytes is then poorer and there are more false results than in the case of a closed, conventional dispenser. This is a result of ambient temperature, atmospheric pressure, and air humidity (3). The detection of tributhylphosphate has demonstrated that when using the open sample inlet detection is 0.980 mg/L; when closed it is 0.196 mg/L; and when closed with a solid-phase microextraction (SPME) dosing system it is 0.0098 mg/L(31). When using SPME, the introduction of oxygen, present in air, into the chromatograph can unfavorably affect sorptive fibers at high temperatures. In this case the use of nitrogen is recommended.
Solids, such as explosives or pesticides, can be analyzed by pyrolysis chromatography or as solutions and aerosols. For this purpose, the thermal solid sample introduction (TSSI) was developed (19,29). In this solution, the solid aerosol particles are stopped on the filter. After heating the filter, the resulting volatile compounds are transferred to the carrier gas and retained in the trap of a six-port valve. The analytes thermally desorbed from the trap are introduced into the chromatographic column. From the solutions of solids such as explosives, the first solvent can be vaporized and subsequently analyzed substances are introduced into the device, as in the case of aerosols (24).
The effectiveness of combining GC and IMS was demonstrated by analyzing liquid–ignitable materials (for example, petrol, solvents) and burnt carpets previously saturated with these liquids. Analyses were performed with GC and IMS alone or in combination (32). Samples were collected for analysis using headspace (HS) SPME. Using GC–DMS, correct results were obtained for 80% to 100% of the samples.
Analysis of exhaled gases is commonly used for diagnosing a variety of human diseases. Gas exhaled from the lungs may contain hundreds of volatile organic compounds, inorganic low-molecular-weight chemical compounds, and low volatile compounds such as isoprostanes, cytokines, and leukotrienes (18,33). Knowing the composition of exhaled gases can be helpful in helping to diagnose diseases of the lungs, liver, and gastrointestinal tract as well as diabetes and sepsis. This type of diagnosis is non-invasive and painless. Such an analysis using only IMS is difficult and the interpretation of analytical signals can be uncertain. This is because the result of IMS analysis is highly influenced by the presence of water in the sample and the carrier and the drift gas. Even if a single substance is introduced into the IMS spectrometer, the shapes of the drift time spectra will vary for different water contents. The admixture of water in the drift gas changes the position of the peak in the drift time spectrum. Increasing the water content in the carrier gas introduced to the reaction section generally lowers the efficiency of ionization. Selective removal of water vapor before direct analysis with IMS is difficult and can lead to loss of certain components of the sample (that is, the exhaled mixture). The addition of a chromatographic column allows for the separation of water vapor from other components. As a result, particular components subsequently enter the IMS and the water does not interfere with the identification of these components.
As well as understanding the natural metabolites produced in the human body, knowledge of the metabolism of drugs (for example, anaesthetics) is also important. Metabolic processes can be studied by analyzing exhaled chemicals (34). In this case, GC–IMS analysis is performed by introducing exhaled gas into the analytical system using a mouthpiece. The gas is introduced to the PTFE vessel, which is connected to a sampling valve with an unheated sample loop and a capacity of approximately 10 mL. To obtain a continuous, constant flow of exhaled air at the outlet of the dosing valve, a miniature pump suction is applied. Instruments for the analysis of exhaled gases are small and can be used in a hospital ward at the bedside.
Technologies that allow for the detection of characteristic metabolites emitted by the human body can be used in search and rescue, for example, to locate humans trapped under debris (35). Similar technologies might also be useful in the early detection of the presence of toxins or chemicals after the release of biological weapons.
One of the methods that can be applied in this case is pyrolysis of biological material (28). Aerosols containing a Gram-positive and Gram-negative bacteria and viruses were tested with portable Py–GC–IMS system. Analyzed materials, suspended in water, were dried at 120 °C and then pyrolyzed by heating to 400 °C in nitrogen for 7 s. The resulting volatile decomposition products were separated in a 4 m x 0.5 mm column at 40 °C to 140 °C with a ramp rate of 120 °C/min. The separated substances were detected using the IMS. Some of these substances were biomarkers that enabled determination of the class of bacteria. For example, 2-pyridinecarboxamide can be an indication of the presence of Gram-positive bacteria and 3-hydroxymyristic acid is indicative of Gram-negative bacteria.
Using GC–IMS coupled to MCC, the volatile metabolites released during propagation of the bacteria Escherichia coli (E. coli) were detected (17). They were analyzed with the introduction of samples through six-port valve with the dynamic HS and were identified as ethanol, acetone, heptan-2-one, and nonan-2-one. It was possible to determine the relationship between the amount of biomass containing E. coli and the concentration of metabolites in the HS. The ability to analyze the composition of biological substances is important not only because it relates to the potential use of biological weapons, but this could also be applied to bioprocess control, health care, and metabolic engineering (17).
Despite the Convention on the Prohibition of the Development, Production, Stockpiling, and Use of Chemical Weapons and on their Destruction, much attention is still paid to the analysis of chemical warfare agents (CWA) and the products of their decomposition (24). Analysis of CWA in the field is difficult because they can appear in small concentrations and often in the presence of many other chemical substances. Separate determination of retention time and the drift time may be difficult. The combination of good resolution GC with the good detectability of the IMS is therefore advantageous.
For the field analysis of water contaminated with tributyl phosphate, a surrogate CWA, GC–IMS with a sample introduction using SPME with fiber-coated 65-µm polydimethylsiloxane (PDMS) containing divinylbenzene particles was applied. In this case, the column used was a 6 m x 0.53 mm, 0.25-µm PDMS. The column temperature began at 30 °C for 5 s and then was increased to 140 °C at a rate of 120 °C/min, with this temperature held for 5 s. Using this temperature program, the separation time of the sample components was 65 s and cooling to the initial temperature took 60 s (31).
IMS has long been used for the detection of explosives. The coupling of IMS to GC offers new opportunities in this field. This has been demonstrated on five explosive substances detected in the presence of four interferents (23). Analyzing 100 combinations of these substances with IMS alone produced 21 false positive responses. When the analysis was performed by GC–IMS, there was only one false positive result.
A regular gas chromatograph combined with DMS was used to analyze nitroglycerin (NG), dinitrotoluene (DNT), dinitrobenzene (DNB), trinitrotoluene (TNT), and pentaerythritol tetranitrate (PETN). For separation of the explosives, a column 5-m long with OV-1 phase was used in a temperature range of 100 °C to 200 °C. Samples were dosed in acetonitrile and the air flowing through the DMS was doped with vapors of water, acetone, methylene chloride, and propanol. The addition of methylene chloride increased the dependence of ion mobility on the electric field three- to sixfold. This allowed for better separation of the ions in the explosives that were detected in 1 s at the low parts-per-billion range (36).
One of the most important tasks in environmental protection is the analysis of volatile organic compounds (VOCs). VOCs have been analyzed in ground water contaminated with gasoline using static headspace (SHS) (37). A 60 m x 0.25 mm, 0.25-µm column with a stationary phase of 5% diphenyl-95% PDMS was used for this purpose. DMS was used as a detector. The same seven VOCs-benzene, toluene, ethylbenzene, m-xylene, p-xylene, o-xylene, and 1,2,4-trimethylbenzene-were found to be present in a number of gasolines. Their detection in water may provide evidence of gasoline pollution. Detectability is dependent on the operating parameters of DMS and may be present at subnanogram levels (37).
Neither IMS nor GC gives full information for the precise identification of a substance. Attempts are therefore being made to indirectly identify the substance. For example, at the nanogram- to picogram-per-liter level, analysis of a large group of VOCs using MCC–IMS was performed (16). A mixture of these compounds was separated using a 20-cm MCC. Measured retention times and mobilities were compared with the results obtained using a standard capillary column and MS. Certain mathematical dependencies between unidentified signals received by MCC–IMS and by GC–MS were found. The aim is to obtain a database that enables identification of VOCs in biological and medical analysis (16).
One highly toxic VOC is formaldehyde, which, along with other VOCs, is present in wood products glued together using urea-formaldehyde resins (22). The aldehyde was analyzed by HS sampling using a SPME method by its derivatization on the sorption fiber using o-(2,3,4,5,6-pentafluorbenzyl)hydroxylamine. For this analysis, a commercial gas chromatograph connected to a miniature DMS was applied.
Methyl tert-butyl ether and BTX (benzene, toluene, and xylene) separated in MCC with a nonpolar stationary phase can be detected with IMS at 3 pg/L in nitrogen and the microgram-per-liter level in water (38). The total analysis time was <90 s.
In a mixture of substances that cause sock malodor, 32 volatile, mainly ammonia- and sulfur-related compounds were identified using SHS and MCC GC–IMS (39).
A miniature GC–DMS instrument has also been used to detect natural contaminants in wines. The volatile components of wines were analyzed using a dynamic HS with sorption of analytes and their subsequent thermal desorption. Undesirable substances in wine were detected at concentrations below the concentrations identified by humans (25).
The use of the HS technique has also allowed the identification of some components of olive oil. Some of these components were also identified during the classification of 49 samples of Spanish olive oils according to commercial grade, using MCC GC–IMS (40). The results were more precise and obtained in a shorter time than those obtained by UV–IMS. During the analysis of the volatile compounds in virgin olive oil, better selectivity was achieved by using CC versus MCC (41).
GC–IMS and UV–IMS were compared during analysis of the changes in the composition of heat transfer fluids used in thermosolar plants (42). UV–IMS can be used for screening of the tested samples; however, GC–IMS allows for the selective detection of benzene and phenol, which are products of decomposition of the heat transfer fluids (43).
SPME is used in many analytical techniques as a method of sample introduction into detectors. For the purposes of GC–IMS systems, a new kind of coating for SPME was developed. The new fiber was tested on organophosphorus pesticides such as diazinon and fenthion and good separation and possible quantitative determination of these pesticides in real samples were achieved (44).
A needle trap device coupled with a portable handheld IMS connected with micro-GC was used for on-site quantitative analysis of α-pinene, limonene, and acetone (27). Quantitative analysis was performed in 330 s with a low limit of detection.
Chromatography combined with IMS is also used in space research (for example, in the Rosetta Comet Mission [45] and the International Space Station [46,47]). Between 2001 and 2009, GC–IMS was used for the analysis of VOCs in the air on the International Space Station. From 2009 to 2012, three models of GC–DMS, which were much smaller and lighter than GC–IMS, were subsequently used. VOCs from the air were trapped in the sorption tube filled with graphitized carbon black and carbon molecular sieve. After heating the tube to 300 °C, the analytes desorbed and entered the column. The column had dimensions 15 m x 0.25 mm, 1.4-µm and the stationary phase was 6% cyanopropyl/phenyl and 94% PDMS. The column temperature ranged from 35 °C to 150 °C as different temperature programs were applied. The 1 mL/min gas flow through the column was increased by the makeup gas to 350 mL/min, because this value is required for the spectrometer. The air recirculation was applied and the gas leaving the apparatus was thoroughly cleaned and dried on carbon molecular sieves. Analyses performed in the space station were controlled by parallel sampling to vacuum vessels, with sample analysis by GC–MS performed later on Earth.
Connecting Ion Mobility Spectrometers to Liquid Chromatographs
Coupling IMS detectors with GC is relatively easy because of the fact that the sample is in the gas state during the analysis process. When high performance liquid chromatography (HPLC) is coupled with IMS (48–52), the analyte in the column is in the liquid phase. Therefore, it is necessary to change the sample state from liquid to gaseous. For this purpose electrospray ionization (ESI) is often used, what results in simultaneous ionization of the sample components. In such systems the analyzed solution after the ionization of its components is desolvated (solvent evaporation). The remaining stages of the detection process are the same as in standard IMS. LC–ESI-IMS systems allow for detection of low volatile organic compounds.
In addition to simple LC–IMS connections, LC–IMS-MS connections are also used. In such systems, the sample components are preseparated in the chromatographic column. Because the IMS detector is typically working at atmospheric pressure and a high vacuum is inside the MS instrument, coupling IMS with MS requires the use of a vacuum system with adequate capacity. Ions from IMS to MS are introduced through a pinhole and the area where there is indirect pressure. The qualitative results obtained from DT IMS and MS are not independent. Molecules with larger mass (m/z) have generally lower mobility. In the case of DMS detectors the peak positions in the compensation voltage spectrum are weakly correlated with the mass of the ions. Because of this, DMS detectors are often used as ion filters to work with mass spectrometers.
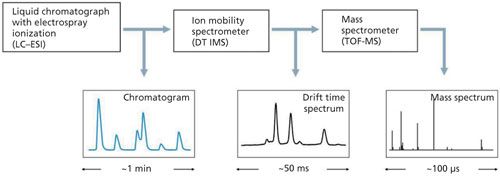
The LC–IMS-TOF-MS system (Figure 4), where the multivariate separation of components of the analyzed sample takes place, is used in the analysis of nonvolatile compounds which is difficult in other techniques. Proteomic studies are conducted using such systems to distinguish peptides in which mass difference occurs at the sixth place after the decimal point. Choosing the type of mass spectrometer is critical for correct working of the entire LC–IMS-MS system. At the same time, the speed of operation of the individual elements is very important. In the LC–DT IMS-TOF-MS system, the width of the peaks from LC are of the order of single seconds. Recording the drift time spectrum in the DT IMS system takes about 50 ms, while measuring the mass spectra of the TOF-MS system takes about 100 µs. This matching of the elements allows for the 3D analysis without loss of information.
Figure 4: Ion mobility spectrometer cooperating with liquid chromatograph and mass spectrometer.
Conclusion
GC–IMS and LC–IMS allow for the collection of more complete analytical information than the use of an ion mobility spectrometer alone. Interest in connecting IMS to GC and especially to LC is now growing. Increasingly, this also applies to combining IMS to other chromatographic techniques and even thin-layer chromatography (TLC) (53). The history of coupling MS to chromatography repeats. First, GC–MS instruments were designed and then HPLC–MS constructions appeared. The issues and possibilities of combining ion mobility spectrometers with chromatographs are examined and these devices are more frequently used to solve different, inter alia difficult, analytical problems.
IMS offers a very low limit of detection. The construction and functionality of IMS devices are not as complicated as MS instruments. IMS devices can be small and light and consume low amounts of energy. All chemicals that can be analyzed with GC may also be analyzed using GC–IMS. The technical parameters of GC–IMS instruments make them suitable for the analysis of many chemicals in field conditions.
References
- A.B. Kanu and H.H. Hill Jr., J. Chromatogr. A1177, 12–27 (2008).
- M.J. Cohen and F.W. Karasek, J. Chromatogr. Sci.8, 330–337 (1970).
- G.A. Eiceman, Z. Karpas, and H.H. Hill Jr., Ion Mobility Spectrometry, 3rd ed., (CRC/Taylor & Francis, Boca Raton, USA, 2013).
- J. Stach and J.I. Baumbach, Int. J. Ion Mobil. Spec.5(1), 1–21 (2002).
- A.A. Shvartsburg, Differential Ion Mobility Spectrometry: Nonlinear Ion Transport and Fundamentals of FAIMS (CRC Press, Boca Raton, USA, 2009).
- R. Guevremont, J. Chromatogr. A 1058, 3–19 (2004).
- B.B. Schneider, E.G. Nazarov, F. Londry, P. Vouros, and T.R. Covey, Mass Spectrom. Rev. DOI: 10.1002/mas.21453.
- J. Puton, A. Knap, and B. SiodÅowski, Sensors Actuat. B-Chem. 135, 116–121 (2008).
- E.V. Krylov, Int. J. Mass Spectrom. 225, 39–51 (2003).
- G.A. Eiceman, B. Tadjikov, E. Krylov, E.G. Nazarov, R.A. Miller, J. Westbrook, and P. Funk, J. Chromatogr. A 917, 205–217 (2001).
- R.A. Miller, E.G. Nazarov, G.A. Eiceman, and A.T. King, Sensors Actuat. A 91, 301–312 (2001).
- F.W. Karasek and R.A. Keller, J. Chromatogr. Sci. 10, 626–628 (1972).
- S. Oller-Moreno, G. Singla-Buxarrais, J.M. Jiménez-Soto, A. Pardo, R. Garrido-Delgado, L. Arce, and S. Marcoa, Sensors Actuat. B-Chem217, 13–21 (2015).
- E. Aguilera-Herrador, S. Cárdenas, V. Ruzsanyi, S. Sielemann, and M. Valcárcel, J. Chromatogr. A 1214, 143–150 (2008).
- M.T. Jafari, M. Saraji, and H. Sherafatmand, Anal. Chem. 84(22), 10077–10084 (2012).
- M. Jünger, B. Bödeker, and J.I. Baumbach, Anal. Bioanal. Chem. 396, 471–482 (2010).
- S. Maddula, L.M. Blank, A. Schmid, and J.I. Baumbach, Anal. Bioanal. Chem.394, 791–800 (2009).
- J.I. Baumbach, J. Breath Res. 3, 1–16 (2009).
- S. Hajialigol, S.A. Ghorashi, A.H. Alinoori, A. Torabpour, and M. Azimi, J. Chromatogr. A 1268, 123–129 (2012).
- J. Luong, R. Gras, H.J. Cortes, and R.A. Shellie, Int. J. Ion Mobil. Spec. 15, 179–187 (2012).
- J.I. Baumbach, S. Sielemann, and P. Pilzecker, J. Ion Mobil. Spec. 3, 28–37 (2000).
- A. Schumann, C. Lenth, J. Hasener, and V. Steckel, Int. J. Ion Mobil. Spec. 15, 157–168 (2012).
- G.W. Cook, P.T. LaPuma, G.L. Hook, and B.A. Eckenrode, J. Forensic Sci. 55(6), 1582–1591 (2010).
- C. Kwan, A.P. Snyder, R.P. Erickson, P.A. Smith, W.M. Maswadeh, B. Ayhan, J.L. Jensen, J.O. Jensen, and A. Tripathi, IEEE Sens. J. 10(3), 451–460 (2010).
- M. Camara, N. Gharbi, A. Lenouvel, M. Behr, C. Guignard, P. Orlewski, and D. Evers, J. Agric. Food Chem.61, 1036–1043 (2013).
- G.R. Lambertus, C.S. Fix, S.M. Reidy, R.A. Miller, D. Wheeler, E. Nazarov, and R. Sacks, Anal. Chem.77, 7563–7571 (2005).
- N. Reyes-Garcés, G.A. Gómez-Ríos, É.A.S. Silva, and J. Pawliszyn, J. Chromatogr.A1300, 193–198 (2013).
- A.P. Snyder, J.P. Dworzanski, A. Tripathi, W.M. Maswadeh, and C.H. Wick, Anal. Chem.76, 6492–6499 (2004).
- S.A. Ghorashi, A.H. Alinoori, and S. Hajialigol, Microelectr. J.45(12), 1634–1640 (2014).
- H. Borsdorf and T. Mayer, Talanta83, 815–822 (2011).
- R.P. Erickson, A. Tripathi, W.M. Maswadeh, A.P. Snyder, and P. A. Smith, Anal. Chim. Acta556, 455–461 (2006).
- Y. Lu and P.B. Harrington, Anal. Chem. 79, 6752–6759 (2007).
- A.V. Guamán, A. Carreras, D. Calvo, I. Agudo, D. Navajas, A. Pardo, S. Marco, and R. Farré, J. Chromatogr. B881, 76–82 (2012).
- A.-E. Kreuder, H. Buchinger, S. Kreuer, T. Volk, S. Maddula, and J.I. Baumbach, Int. J. Ion Mobil. Spec. 14, 167–175 (2011).
- W. Vautz, R. Slodzynski, C. Hariharan, L. Seifert, J. Nolte, R. Fobbe, S. Sielemann, B.C. Lao, R. Huo, C.L. Thomas, and L. Hildebrand, Anal. Chem.85(4), 2135–2142 (2013).
- G.A. Eiceman, E.V. Krylov, and N.S. Krylova, Anal. Chem.76, 4937–4944 (2004).
- F. Liang, K. Kerpen, A. Kuklya, and U. Telgheder, Int. J. Ion Mobil. Spec.15, 169–177 (2012).
- S. Sielemann, Z. Xie, H. Schmidt, and J.I. Baumbach, Int. J. Ion Mobil. Spec.4(2), 69–73 (2002).
- C.J. Denawaka, I.A. Fowlis, and J.R. Dean, J. Chromatogr. A1338, 136–148 (2014).
- R. Garrido-Delgado, F. Mercader-Trejo, S. Sielemann, W. de Bruyn, L. Arce, and M. Valcárcel, Anal Chim Acta.696, 108–115 (2011).
- R. Garrido-Delgado, Maria del Mar Dobao-Prieto, L. Arce, and M. Valcarcel, Food Chem. 187, 572–579 (2015).
- L. Criado-García, R. Garrido-Delgado, L. Arce, F. López, R. Peón, and M. Valcárcel, Microchem. J.121, 163–171 (2015).
- L. Criado-García, R. Garrido-Delgado, L. Arce, F. López, R. Peón, and M. Valcárcel, Talanta144, 944–952 (2015).
- M.T. Jafari, M. Saraji, and H. Sherafatmand, Anal Chim Acta.814, 69–78 (2014).
- M. Grabka, P. Zukowski, and Z. Witkiewicz, ABiD17, 69–77 (2012).
- T. Limero, E. Reese, P. Cheng, and J. Trowbridge, Int. J. Ion Mobil. Spectrom.14, 81–91 (2011).
- T. Limero, E. Reese, W.T. Wallace, P. Cheng, and J. Trowbridge, Int. J. Ion Mobil. Spec. 15, 189–198 (2012).
- K.F. Tadjimukhamedov, J.A. Stone, D. Papanastasiou, J.E. Rodriguez, W. Mueller, H. Sukumar, and G.A. Eiceman, Int. J. Ion Mobil. Spectrom.11, 51–60 (2008).
- Y.M. Ibrahim, E.S. Baker, W.F. Danielson III, R.V. Norheim, D.C. Prior, G.A. Anderson, M.E. Belov, and R.D. Smith, Int. J. Mass Spectr. 377, 655–662 (2015).
- J.P. Williams, M. Kipping, and J.P.C. Vissers, G.I.T. Laboratory Journal 16(3–4), 36–38 (2012).
- G. Astarita, G. Paglia, and K. Yu, LCGC Europe 28(9), 520–525 (2015).
- K.L. Crowell, G.W. Slysz, E.S. Baker, B.L. LaMarche, M. E. Monroe, Y.M. Ibrahim, S.H. Payne, G.A. Anderson, and R.D. Smith, Bioinformatics29(21), 2804–2805 (2013).
- V. Ilbegi and M. Tabrizchi, Anal. Chem. 87, 464–469 (2015).
Zygfryd Witkiewicz is a professor of analytical chemistry at the Institute of Chemistry of the Military University of Technology in Warsaw, Poland; he is also associated with Jan Kochanowski University in Kielce, Poland. His research activity is focused on the analysis of environmental organic pollutions with some interest in chemical warfare agents. In his investigations he uses mainly GC but also HPLC and TLC. He is also involved in sample preparation methods for chromatographic analysis. As an university teacher he has written several textbooks concerning the fundamentals of chromatographic and electromigrating techniques. He is one of two editors of a five-language vocabulary on chromatography, which was released in 2016.
Urszula Gaik (M.Sc.Eng.) is a PhD student at the Military University of Technology in Warsaw, Poland. Her research is focused on the use of IMS. The application of nitrogen oxides as dopants to carrier gas in IMS is her main interest.
Edyta Budzynska (M.Sc.Eng.) is a PhD student at the Military University of Technology in Warsaw, Poland. Her research is focused on the use of ion mobility spectrometers as detectors in gas chromatography. Optimization of GC–IMS systems is her main interest.
MirosÅaw Maziejuk is a researcher at the Military Institute of Chemistry and Radiometry (since 1985) and he has been in charge of the radiometry and camouflage department since 2010. He earned his PhD at the Military University of Technology in 1995. Since 1996 he has been working on developing detection techniques used for determination of toxic substances. He is a specialist in IMS and DMS.
JarosÅaw Puton is a researcher and lecturer at the Military University of Technology in Warsaw, Poland. His main research topic is IMS. He is interested in investigations of the quantitative aspects of analysis conducted with IMS including impact of dopants on the detection course. He is also the author of papers related to transport phenomena in IMS, for example, explaining the transmissions of ion through the shutter and aperture grids in IMS detectors. Dr. Puton earned his PhD Diploma in Technical Sciences (1980) at the Military University of Technology and his Habilitation (2016) at Gdansk University of Technology.
This article was originally published in LCGC Europe in June 2016. This version has been modified slightly.

Synthesizing Synthetic Oligonucleotides: An Interview with the CEO of Oligo Factory
February 6th 2024LCGC and Spectroscopy Editor Patrick Lavery spoke with Oligo Factory CEO Chris Boggess about the company’s recently attained compliance with Good Manufacturing Practice (GMP) International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) Expert Working Group (Q7) guidance and its distinction from Research Use Only (RUO) and International Organization for Standardization (ISO) 13485 designations.