Lean, Green Food-Testing Machines: How Innovations in Microflow LC-MS Can Improve Food Safety Test Methods
This article describes how a food testing laboratory can transition routine high performance liquid chromatography (HPLC) methods to microflow LC for improved sensitivity, throughput, and robustness of analysis.
Food testing labs work endlessly to test our food supply for hazardous chemicals and contaminants to ensure human and animal safety. For a routine food testing laboratory, this typically means they must prepare hundreds of food samples daily for analysis, analyze those samples for hundreds of contaminants, and do it all with a fast turnaround of results. In recent years, innovations in microflow liquid chromatography (LC) coupled to mass spectrometry has created opportunities for laboratories to do more with less — more sensitivity, higher throughput, and more robustness in their analyses but in less time, with lower cost, and with less hazardous solvent consumption. For a high-throughput food testing laboratory, microflow LC coupled to mass spectrometry is an innovation that can push routine food testing analysis to the next level. Here, we describe how a food testing laboratory can transition routine high performance liquid chromatography (HPLC) methods to microflow LC and show performance results that showcase improved sensitivity, throughput, and robustness of analysis, with the added benefits of reduced solvent waste and overall analysis costs.
Food is a vital part of life, and many food scientists and chemists work hard each day to test food products and ingredients for safety and quality. From pesticides to antibiotics, allergens to natural toxins, and so many others, the number of contaminants that can often be found in foods, ingredients, and assorted consumer products continues to increase.
As the demand to test more products for more types of contaminants increases, the workload on food testing laboratories increases as a direct result. With this increase, food testing scientists are continuously looking for new methods and technologies to improve their workflows. From more efficient and effective ways to extract contaminants and remove matrix components in sample preparation, to faster and higher quality chromatography as well as more sensitive and selective detection approaches, food testing scientists are in constant pursuit to test more samples with faster turnaround of results with the highest level of accuracy and reliability.
As an added pressure, modern analytical equipment can sometimes require an abundance of resources, including consumable resources such as solvents, chemicals, and disposable equipment as well as personnel resources such as instrument run management and maintenance. These resource requirements can also put a strain on food testing laboratories, which endlessly strive to keep their cost per analysis low and their efficiency to produce results high.
Fortunately, advances in modern analytical instrumentation can relieve some of these strains on routine food testing laboratories. From better sample preparation approaches to new liquid chromatography coupled to tandem mass spectrometry (LC–MS-MS) technology, food testers now have better accessibility to techniques that will save them time, save them money, and give them better results than ever before.
LC has been a preferred methodology for food testing laboratories for many years, allowing ideal separation, identification, and detection of contaminants in complex food samples. Routine food testing laboratories run samples around the clock, which results in considerable resource consumption, excessive expenses in consumable costs, and considerable production of organic solvent waste.
New innovations in LC technology, such as microflow LC, present laboratories with a more efficient alternative to higher-flow LC methods. Microflow LC requires significantly less solvent usage and less analytical sample consumption, without sacrificing any of the performance features of normal-flow LC, making it substantially more cost and time efficient for a high-throughput testing laboratory while still providing the best analytical results. Furthermore, the reduced solvent and chemical consumption has a reduced environmental impact, making microflow LC a leaner, "greener" choice for environmentally conscious laboratories.
In this article, we present a research study that shows how routine food testing methods, such as pesticide analysis in spices, can be transferred from traditional LC–MS-MS to microflow LC–MS-MS to save laboratories time, money, and resources without sacrificing analytical performance and quality of results.
Material and Methods
Two food samples were prepared and analyzed on both a conventional LC–MS-MS system and on a microflow LC–MS-MS system. The food samples (chili powder and fresh basil, two notoriously difficult matrices for food testing laboratories) were prepared using a standard QuEChERS (quick, easy, cheap, effective, rugged, and safe) approach (1). Briefly, 5 g of herb or spice was homogenized, hydrated with water (10 mL), and extracted with acidified acetonitrile (0.05% acetic acid in acetonitrile, 10 mL). QuEChERS salts (magnesium sulfate) were added to the sample, and the sample tube was rigorously shaken and centrifuged. An aliquot of the supernatant (6 mL) was mixed with dispersive solid-phase extraction (SPE) cleanup solids (PSA and C18), shaken vigorously, and centrifuged. The resulting supernatant was diluted 1:10 with water before analysis. The Sciex iDQuant standards kit (AB Sciex) for pesticide analysis (containing approximately 200 pesticide compounds) was used to prepare the calibration solvent standards as well as to prepare pesticide-spiked matrix samples.
Two LC–MS-MS systems were used for the comparative evaluation, one with the MS system coupled to a conventional high performance liquid chromatography (HPLC) system and the other to a microflow LC system. For the conventional HPLC system, a 5.0 mm × 4.6 mm, 2.6-μm Kinetex core–shell column (Phenomenex) was used with temperature set at 50 °C. For the MS-MS set up (QTRAP 4500, AB Sciex), a standard 100-μm i.d. Turbo V electrospray ionization (ESI) probe (AB Sciex) was used, with a source temperature of 550 °C, nebulizer gas (GS1) setting of 50 psi, heater gas (GS2) setting of 60 psi, and source voltage of 5500 V. For the microflow LC system, a 5.0 mm × 4.6 mm Halo C18 column (Advanced Materials Technology) was used with the temperature set at 50 °C. For the MS-MS setup, a microflow LC hybrid electrode (50 μm i.d.) (2) probe was used, with a source temperature of 450 °C, nebulizer gas (GS1) setting of 25 psi, heater gas (GS2) setting of 25 psi, and source voltage of 5500 V.
Identical mobile phases and gradients were used for the preliminary evaluation to compare conventional LC and microflow LC performance. The mobile-phase gradient of 98% water to 95% methanol (containing 2 mM ammonium acetate and 0.1% formic acid modifiers) had a 15-min total run time. The injection volume on both systems was 2 μL, and the flow rates were 400 μL/min and 40 μL/min for the conventional HPLC and microflow LC systems, respectively.
The Scheduled MRM algorithm was used for data acquisition to maximize the number of data points across each peak for the 125 pesticides assessed in the study (two MRM transitions per pesticide were used for a total of 250 MRM transitions).
Data were processed and analyzed using Analyst software version 1.6 and MultiQuant software version 2.1 (AB Sciex).
The following performance criteria were used to evaluate and compare the two instruments: peak shape and sensitivity, linearity, injection carryover, robustness, and solvent consumption.
Results and Discussion
The aim of this study was to assess the applicability of microflow LC for routine food testing applications and compare the sensitivity and performance with a traditional, higher-flow method already established for pesticide analysis in food samples. In this study, the chromatography was not optimized for speed, although it should be noted that the methods, particularly the microflow LC method, could be optimized for faster chromatography and reduced run times.
Peak Shapes and Sensitivity
To evaluate the peak shape and sensitivity of the two systems, a pesticide standard containing 2 ppb each of more than 200 common pesticides was injected in each system. The extracted ion chromatograms of one MRM transition for two different pesticides are shown in Figure 1. The results show excellent peak shapes on both systems.
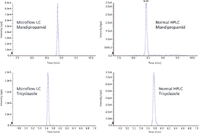
Figure 1: Comparison of the peak shapes of two pesticides using microflow LC (left-hand panel) and conventional LC (right-hand panel).
Signal-to-noise ratios (taken directly from MultiQuant software) were used to assess the relative sensitivity of the two systems, using selected pesticides that were eluted at various times throughout the run. A sensitivity comparison of nine pesticides is shown in Table I. The results show an increase in the MS instrument response when the microflow LC is used, with little increase in noise, resulting in signal-to-noise increases ranging from threefold to 10-fold relative to conventional flow LC.

Table I: Comparison of LCâMS sensitivities of nine pesticides spanning the analytical run for the conventional LC vs. the microflow LC system
A benefit of the added sensitivity of microflow LC is the ability to dilute samples further to reduce matrix effects on the results and also to potentially reduce injection volumes, lowering the amount of sample loaded onto the chromatography column, which could improve the robustness of the analysis, extend the life of the column, and reduce the maintenance required for the LC–MS-MS system.
Linearity
Linear response of analytical instrumentation is highly important for food testing applications because of the need for accurate quantitation over a wide range of concentrations. Conventional LC methods typically show excellent linear range; therefore, we tested the microflow LC system to confirm that the linear response is preserved using this front-end chromatography system. The linearity was assessed for a pesticide concentration range of 0.2–100 ppb with a linear regression fit, and the r value obtained for the majority of the pesticides was greater than 0.999. The results confirmed that linearity for quantitative LC–MS is preserved with the microflow LC chromatography.
Injection Carryover
A big concern in LC analysis, and an important feature of suitable LC systems and conditions, is that carryover between injections must be minimal to nonexistent. We wanted to verify that the carryover using the microflow LC front end was suitable and comparable to conventional LC systems, which typically are listed as having carryover values of 0.05% or less. To assess the carryover, a 100 ppb pesticide mix standard (which produces a saturated response in the mass spectrometer for most pesticides) was injected, and the carryover from the injection was assessed by evaluating the pesticide responses in a water blank injected immediately following the 100 ppb standard injection. The results of this assessment are shown in Figure 2. It was noted that for the majority of pesticides, no carryover was observed in the water blank, and the overall carryover was estimated at <0.1%.
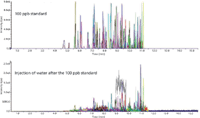
Figure 2: Carryover study showing a 100 ppb pesticide mix standard injection (top panel) and a water blank injection immediately after (bottom panel).
Robustness
Food testing laboratories often put excessive stress on their analytical instruments, running many samples (often samples containing high levels of "dirty" matrix components) around the clock. As a result, instrument robustness is essential as the results obtained in the final run of the batch must be just as reliable as the results obtained in the first run of the batch. To test the robustness of the microflow LC system, repeated injections of unfiltered chili powder extract, a notoriously difficult matrix for food laboratories, were made, totaling more than 150 subsequent injections without changing the column, cleaning the system, or performing any maintenance.
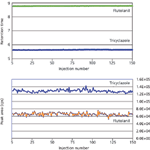
Figure 3: Results showing the retention times and peak areas observed for two pesticides (flutolanil: yellow trace and tricyclazole: blue trace) after repeated injections of a crude spice sample using the microflow LCâMS-MS system. These two pesticides were selected to assess robustness early in the analytical run as well as later in the analytical run.
The retention times and peak areas of the pesticides were assessed for the duration of the 150 injections (Figure 3), and the pressure profile of the microflow LC system was plotted at the first injection and again at the 150th injection (Figure 4).
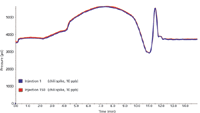
Figure 4: Pressure profile obtained from the microflow LC for the first injection of the spice sequence and the 150th injection of the spice sequence. Results show that the system is highly robust for long runs with "dirty" samples.
The results show that the LC–MS retention times are rock solid for the duration of the 150 injections, with little to no variability observed. These results confirm that fast equilibration times are possible with the microflow LC system, and also verify the benefits of the reduced dead volume of the system. Additionally, peak area responses measured throughout the injection sequence revealed no deterioration in total signal. This result was surprising because with repeated injections of dirty matrix samples, some peak response loss is typically observed. However, it was shown that the robustness of the microflow LC–MS-MS withstands these fluctuations, potentially in part due to the low sample injection volume required for analysis using micro-flow LC.
Solvent Consumption
Results showed that the performance of microflow LC was as good as that of conventional LC, and even better in some cases. Given the reduction in solvent consumption of the microflow LC system (40 μL/min flow versus 400 μL/min flow), we did one final evaluation calculating the relative solvent consumption of the microflow LC system versus the conventional LC system.
With the following assumptions:
- 15-min run time per injection
- Gradient consumes approximately 50% organic phase
- Methanol cost estimate of $1000 per 4 × 4 L case
- Approximately 200 samples injected per day
- Samples run five days per week, 52 weeks per year.
A laboratory could see cost savings of nearly $9000 just in solvent consumption per year (which does not include any additional costs incurred in waste disposal of solvents) by transitioning methods from conventional LC to microflow LC.
Microflow LC also can provide improved separation over normal LC, allowing the potential to reduce run times and greatly improve not only sample throughput, but also provide cost savings in solvent consumption. Figure 5 shows an example of a rapid gradient for the analysis of pesticides in food, where run time is reduced from 15 min per injection to 4 min per injection.
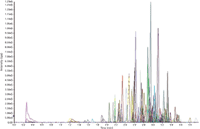
Figure 5: Injection of a 1 ppb pesticide standard with a rapid microflow LC gradient.
Conclusions
This study has demonstrated the capabilities of microflow LC–MS–MS for routine testing of contaminants in food samples. With the increasing demand on food testing laboratories to test more samples (and a larger variety of matrices) for more compounds with better performance, while also improving throughput and efficiency, it is clear that new techniques such as microflow LC can have a strong impact in routine food-testing laboratories. With improved sensitivity and throughput, without any sacrifice in analytical performance and reliability in results, microflow LC is a viable alternative to conventional LC for food laboratories striving to improve their methods while also improving their bottom line.
Also, with the reduced solvent consumption associated with microflow LC, environmentally conscious scientists can feel better about using more sustainable technology with reduced environmental impact. As these results show, becoming a lean, "green" food-testing machine can now be as easy as a simple swap from LC to microflow LC, and the graduation to the next generation of sustainable LC–MS-MS technology.
Stephen Lock is an application manager for EMEA with AB Sciex in the United Kingdom.
Lauryn Bailey is a global marketing manager for the food & environmental markets with AB Sciex in Framingham, Massachusetts. Direct correspondence to: lauryn.bailey@absciex.com.
References
(1) M. Anastassiades et al., J. AOAC Int. 86, 412–431 (2003).
(2) K. Mriziq et al., Technical Note Eksigent (2011) # 4590211-01.

Synthesizing Synthetic Oligonucleotides: An Interview with the CEO of Oligo Factory
February 6th 2024LCGC and Spectroscopy Editor Patrick Lavery spoke with Oligo Factory CEO Chris Boggess about the company’s recently attained compliance with Good Manufacturing Practice (GMP) International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) Expert Working Group (Q7) guidance and its distinction from Research Use Only (RUO) and International Organization for Standardization (ISO) 13485 designations.