Microwave Digestion of Pharmaceutical Finished Products and Ingredients for Upcoming USP Method 233
Application Notebook
Current USP Method 231 "Heavy Metals" was introduced in 1905 for determining heavy metal impurities in pharmaceutical drug products. The procedures of the method have several limitations, which produce results that are nondiscriminatory, difficult to reproduce, and qualitative, or at best, semi-quantitative.
Current USP Method 231 "Heavy Metals" was introduced in 1905 for determining heavy metal impurities in pharmaceutical drug products. The procedures of the method have several limitations, which produce results that are nondiscriminatory, difficult to reproduce, and qualitative, or at best, semi-quantitative. For these reasons, the United States Pharmacopeia will be replacing USP Method 231 with USP Method 232 "Elemental Impurities Limits" and USP Method 233 "Elemental Impurities Procedures" in September 2013. USP Method 233 requires the use of current analytical instrumentation including a closed vessel microwave digestion system for sample preparation and an ICP-OES or ICP-MS for analysis. The CEM MARS™ 6 microwave digestion system with EasyPrep™ vessels was used to prepare samples for analysis by ICP-OES. The results were compared to allowable limits stated in USP Method 232.

Figure 1: Running at full vessel turntable capacity, the MARS 6 completely digested acetylsalicyclic acid samples (shown above) compared to the partial digestion accomplished in a traditional microwave laboratory digestion system operating under the same parameters.
Experimental
Two pharmaceutical samples were prepared, digested, and analyzed with an ICP-OES. A finished product, acetylsalicylic acid tablets, was ground into a powder prior to use and an ingredient, folic acid, was used without preparation. A 0.50 g sample, 5 mL of concentrated nitric acid, and 5 mL of spiked DI water containing 125 ppb of arsenic, cadmium, lead, and mercury were added to an EasyPrep vessel. Each of the samples and blanks were prepared in triplicate and digested in a single run in a CEM MARS 6 with EasyPrep vessels. The samples were ramped to 200 °C in 25 min and held at this temperature for 15 min. The digested samples were diluted with DI water to a volume of 25 mL. The samples and three blanks containing 20 mL of DI water and 5 mL of HNO3 with no concentration of arsenic, cadmium, lead, or mercury were analyzed with an ICP-OES.
Results
The average, percent recovery, and RSD for each of the samples are shown in Table I.
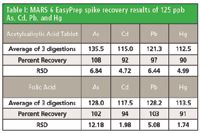
Table I: MARS 6 EasyPrep spike recovery results of 125 ppb As, Cd, Pb, and Hg
The USP Method 233 proposed accuracy is 70–150% and the proposed precision is no more than 20%. The results from the digestion and analysis of acetylsalicylic acid tablets and folic acid show that the volatile elements, arsenic and mercury, had percent recoveries from 90–108% and an RSD of less than 12.2%. The results from the digestion and analysis of the nonvolatile elements, cadmium and lead, had percent recoveries from 92–103% and an RSD of less than 6.5%. The percent recovery and RSD for each element is well within the proposed requirements of USP Method 233.
Conclusions
Microwave digestion provides a fast, accurate, and reliable method for sample preparation prior to elemental analysis. The CEM MARS 6 microwave digestion system with the high-temperature, high-pressure EasyPrep vessels with proprietary vent and reseal technology provide the necessary temperatures and pressures for a complete digestion of pharmaceutical finished products and ingredients without the loss of volatile analytes because of uncontrolled venting, ensuring the accuracy and precision needed to meet the requirements of proposed USP Method 233.
CEM Corporation
PO Box 200, Matthews, NC 28106
tel. 704) 821-7015, (800) 726-3331; Fax: (704) 821-7894
Website: www.cem.com

Accurate Microplastics Analysis in Minutes, Not Hours
April 10th 2024The automated Agilent 8700 LDIR chemical imaging system lets you obtain high-quality images and spectral data faster than ever before. So, you can perform confident large-scale microplastics studies and monitoring activities.
Advancing Research of Plastics in the Environment Using the Agilent Cary 630 FTIR Spectrometer
April 10th 2024Plastic pollution has become a high-priority area of study in recent years due to the increasing prevalence of plastics in the environment. Currently, researchers have a limited understanding of the impact of plastic pollution on human health, how it affects wildlife and their habitats, and its long-term effects on the environment. An important step in overcoming this pressing global environmental issue is the advancement of research relating to the identification of plastic waste and microplastic particles.