Simplifying Mixed-Food Microwave Sample Preparation for ICP-MS Analysis
Low-level analysis of food matrices has placed a demand on manufacturers, testing laboratories, and instrumentation vendors worldwide. Stricter regulations, better analytical instrumentation, and greatly improved sample preparation (preanalytical) techniques have focused efforts to simplify and standardize these analyses. Often overlooked, the preanalytical step determines the quality of the resulting data and requires careful attention to a number of details, including sample size, digestion parameters, and the level of detection needed.
Low-level analysis of food matrices has placed a demand on manufacturers, testing laboratories, and instrumentation vendors worldwide. Stricter regulations, better analytical instrumentation, and greatly improved sample preparation (preanalytical) techniques have focused efforts to simplify and standardize these analyses. Often overlooked, the preanalytical step determines the quality of the resulting data and requires careful attention to a number of details, including sample size, digestion parameters, and the level of detection needed. This article describes the selection of critical factors used in the optimization and simplification of food and mixed-batch food microwave digestion.
Food and contract testing laboratories are in an uphill battle in determining the best approach in processing foodstuffs. Regulatory bodies such as the Food and Drug Administration (FDA), United States Department of Agriculture (USDA), and the European Commission (EC) have each established element concentration limits on food and agricultural products. A recent addition to the methods menu from the FDA provides a guideline for the preanalytical step for microwave sample digestion followed by inductively coupled plasma–mass spectrometry (ICP-MS) analysis of trace metals in food (1). Many publications and the Association of Analytical Communities (AOAC) performance methods have validated microwave digestion as a standard approach to food analysis. In fact, Method 4.7 from the FDA is an example of an internal validation exceeding standards set forth by the AOAC. The USDA has also recently updated their chemistry laboratory guidebook for the analysis of food utilizing inductively coupled plasma–optical emission spectrometry (ICP-OES) and ICP-MS following sample microwave digestion (2). The important feature in many of these methods relates to the choice of analytical tools and sample preparation technique, which leads to the questions: What is the best way to perform sample preparation and which instrument is best for multielement analysis within a laboratory setting?
Sample preparation is given little attention in the overall process for the analysis of trace metals, and for many sample types it turns out to be routine. Normally, scientists simply look at the detection limits for an ICP-OES or ICP-MS system and calculate back from a detection level or interferences to determine the instrument of choice. Unfortunately, with highly organic sample types and the breadth of complexity increasing, the need for a good quality digestion process is more critical. A list of preanalytical contributing factors that can affect the backend analysis includes sample size, acid digestion volume, sample fat and organic content, contamination, throughput, and post-digestion residual carbon content. Each of these will play a role in the methods chosen for processing a food sample.
Larger samples sizes historically have been used because of the limits of analytical detection and sample inhomogeneity. The consideration on what to use for the sample preparation then becomes a choice between ashing and microwave digestion. Ashing is complicated by the loss of critical elements such as Cd, Pb, and Hg along with contamination issues (3). Microwave digestion also historically has been a concern with the lack of vessel temperature and pressure capability for complete digestion of reasonably sized sample amounts. This picture becomes even more opaque with incomplete digestions causing problems for the ICP-OES or ICP-MS analysis with a resulting high residual carbon content (4–6). While ICP-OES still dominates the testing laboratory environment, there is a consistent movement toward ICP-MS with all of the advantages and trace metal regulations it offers (7).
Conventional closed-vessel microwave digestion has been recognized for the past two decades as the most effective technique for the digestion of the widest range of sample types in metals analysis, largely because of its ability to operate at high temperatures and pressures. Because the digestions are in a closed vessel, cross-contamination and the loss of volatile elements are eliminated (8). Additionally, microwave sample preparation techniques have overcome challenges of poor digestion quality by providing better digests with low residual acidity, lower carbon content, and lower concentrations of dissolved solids, a critical feature necessary for back-end ICP-MS and ICP-OES analysis (9). It was recently suggested that an ideal sample preparation procedure would be capable of digesting large sample amounts, provide high throughput, be compatible with multielement analysis, and be safe, green, and easy to use (9). A recent publication illustrates the importance of sample size, lower acid consumption, complete digestion with low residual carbon, and the effects of temperature and pressure with a variety of food samples in a conventional microwave and a new single reaction chamber technology (10). This research goes a long way into the basic understanding of how to think about sample preparation as a process. To further illustrate the completeness of sample preparation, we used a single reaction chamber (SRC) microwave digestion approach for processing mixed-batch and large food samples.
Experimental
Methods and Materials
SRC microwave digestion has been utilized extensively in the last several years. The operation, specification, sequence of operation, and application has been published for further reading (11,12). In short, the SRC system uses a large, prepressurized chamber where multiple independent samples or sample types are digested simultaneously up to 300 °C and 199 bar (including multiple acid combinations for determining the right digestion chemistry). By choosing different size racks to fit inside the chamber, different sample sizes and throughput can be performed depending on internal needs. Prepressurizing the chamber allows a single microwave method to be chosen for all types without the need for extensive method development.
All digestions were developed using an UltraWAVE microwave (Milestone) based on the SRC design. The microwave delivers 1500 W of microwave power to a 1-L stainless-steel reinforced PTFE chamber that can hold sample racks of 5, 15, or 22 vessels depending on sample and acid volume. Direct temperature and pressure control provides direct control of each sample (all samples reach the same temperature and pressure). The experiments described in this article were performed using a 15-position rack with 15-mL disposable glass vials and a five-position rack with quartz vials for large sample sizes. An Agilent 7700x ICP-MS system was used for the analysis of all the elements. The operating conditions for the ICP-MS system were as follows: RF applied power to the torch, 1.55 kW; carrier gas flow, 0.95 L/min; dilution gas flow rate, 0.15 L/min; plasma mode normal, He cell gas flow, 4 mL/min. Operating in He collision mode for samples allowed for routine robust analysis.
Microwave Sample Preparation
ICP-MS analysis of mixed-food samples following single reaction chamber microwave digestion (0.5 g, 4 mL HNO3, 0.5 mL HCl; 30 min to 240 °C with 15 min at 250 °C)
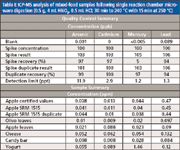
Samples were selected to include a variety of food types along with standardized reference materials. The primary "big four" toxic metal profiles were used to establish a microwave method and instrument baseline numbers. Following this approach, an expanded set of metals was determined using the same microwave method. The final experiment examined a set of samples with sizes greater than 2 g to illustrate the feasibility of processing larger food samples with an identical microwave method established in the previous experiments (expands the capability to use ICP-OES in the well established food industry).
Table II: ICP-MS analysis of mixed-batch sample types following single reaction chamber microwave digestion (0.5 g, 5 mL HNO3; 30 min to 240 C with 15 min at 240 C)
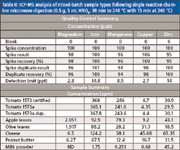
Typical food samples (0.5 g) were placed in disposable glass test tubes and placed in a 15-position rack for microwave operation. The samples were treated with a 4-mL nitric acid and 0.5-mL hydrochloric acid combination (for Cd, Pb, As, and Hg stabilization) or 5 mL of nitric acid for the expanded list of elements and placed in the microwave chamber (note: the PTFE liner contains 110 mL of water, 10 mL of hydrogen peroxide, and 3 mL of sulfuric acid as the microwave load for parameter control). Following prepressurization of the chamber with nitrogen to 40 bar, the samples were digested using the following time–temperature microwave method: Ramp to 240 °C over 30 min, hold at 240 °C for 15 min, cool to 60 °C, and depressurize the chamber for analysis; the total time was 55 min. It should be noted that pressures can reach 100 bar or greater during the course of a digestion and sample decomposition, illustrating one of the critical needs to have a system capable of higher temperatures and pressures for quality digestions. Systems or vessels venting during the course of these conditions would preclude development with the loss of volatile elements at a partial digestion point during a method.
Results
Table III: ICP-MS analysis of large sample sizes following single reaction chamber microwave digestion (2–2.5 g, 10 mL HNO3, 4 mL H2O, 1 mL HCl; 30 min to 240 °C with 15 min at 240 °C)
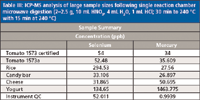
Three separate microwave digestion runs were performed with a variety of food sample types and multiple sample sizes. Each of the microwave methods formed clear digestates with lower acid volumes, which were diluted with water to 25 mL, followed by a 2-mL aliquot diluted to 40 mL, and analyzed using ICP-MS. The first sample set was analyzed for As, Cd, Hg, and Pb. The second sample set was expanded to include Mg, Mn, Cu, Fe, and Zn. Lastly, larger sample sizes were digested and analyzed for Se and Hg. For quality control (QC), an acid blank, spikes, and duplicates were analyzed as part of ongoing instrument calibration verification. Quantitative data, detection limits, and QC data are shown in Tables I, II, and III.
Conclusion
Given the high-throughput nature of testing laboratories, the standardization of the SRC microwave method aligns well with the use of ICP-OES or ICP-MS analysis for all sample types. SRC microwave digestion in food, testing, and manufacturing laboratories for the analysis of trace metals provided a single optimized sample preparation method. To achieve complete digestions for multiple sample types simultaneously and larger same sizes, an optimized temperature of 240 °C was used; disposable glass vials were used to eliminate the need for vessel cleaning in subsequent digestion runs. With a 15-position rack and a 55-min digestion time start to finish, a series of multiple samples can be processed for multielement trace metals. Standardizing the SRC method provided a way to scale the process to larger sample sizes without the need for extensive method development, batching, vessel cleaning, and assembly.
David Gunn is the applications manager at Milestone, Inc., in Shelton, Connecticut. Direct correspondence to: d.gunn@milestonesci.com
References
(1) P. Gray, W. Mindak, and J. Cheung in Elemental Analysis Manual (EAM) for Food and Related Products (U.S. Food and Drug Administration, U.S. Department of Health and Human Services, Silver Spring, Maryland, 2013), section 4.7. Retrieved from
http://www.fda.gov/downloads/Food/FoodScienceResearch/ LaboratoryMethods/UCM377005.pdf.
(2) United States Department of Agriculture (USDA), Determination of Metals by ICP-MS and ICP-OES (Optical Emission Spectrometry) (United States Department of Agriculture, Food Safety and Inspection Service, Office of Public Health Science, Washington, DC, 2013). Retrieved from
http://www.fsis.usda.gov/wps/wcm/connect/b9a63ea1-cae9-423b-b200-36a47079ae49/CLG-TM3.pdf?MOD=AJPERES.
(3) J.S. Barin, B. Tischer, R.S. Picoloto, F.G. Antes, F.E.B. Silva, F.R. Paula, and E.M.M. Flores, J. Anal. At. Spectrom. 29, 352–358 (2013).
(4) M. Wurfels, E. Jackwerth, and M. Stoeppler, Anal. Chim. Acta 226, 1–16 (1989).
(5) P. Fecher and G. Ruhnke, At. Spectrosc. 19, 204–206 (1998).
(6) G. Grindlay, L. Gras, J. Mora, and M.T.C. de Loos-Vollebregt, Spectrochim. Acta Part B 63, 234–243 (2008).
(7) S. McSheehy, M. Hamester, and M. Godula, Food Qual. Saf. (February/March, 2010). Retrieved from
http://www.foodquality.com/details/article/809781/ICP-MS_for_Detecting_Heavy_Metals_in_Foodstuffs.html.
(8) D. Gunn, Spectroscopy supplement: Applications of ICP & ICP-MS Techniques for Today's Spectroscopists 28(s11), 8–16 (2013).
(9) J.A. Nobrega, C. Pirola, L.L. Fialho, G. Rota, C.E.K.M.A. Campos Jordão, and F. Pollo, Talanta 98, 272–276 (2012).
(10) C.C. Mueller, A.L.H. Muller, C. Pirola, F.A. Duarte, E.M.M. Flores, and E.I. Muller, Microchem. J. 116, 255–260 (2014).
(11) T. Michel, Amer. Lab. 42, 32–35 (2010).
(12) T. Michel and S. Hussain, Spectroscopy supplement: Applications of ICP & ICP-MS Techniques for Today's Spectroscopists 27(s11), 30–35 (2012).

Inside the Laboratory – The Petrochronology Group at University of California, Santa Barbara
January 29th 2024In this edition of “Inside the Laboratory,” John Cottle, PhD, a professor of geology at the University of California, Santa Barbara, and a member of Spectroscopy’s Editorial Advisory Board, discusses his group’s most recent work using “laser ablation split steam” analysis to measure elemental concentrations and isotopic ratios in rocks and minerals.