The Detection of Biomarkers and Cocaine in Fingernails Using Attenuated Total Reflectance Fourier Transform Infrared Spectroscopy
Attenuated total reflectance Fourier transform infrared spectroscopy (ATR-FT-IR) provides portable and rapid analysis of biomarkers and drugs within fingernails. Fingernails offer a suitable alternative to traditional biological matrices and provide advantages such as non-invasive collection and requiring small sample sizes. This work utilized ATR-FT-IR for detecting biomarkers and cocaine within fingernails. Fingernails were analyzed initially “as received” to identify biomarkers such as carbohydrates, lipids, and proteins over the range 650–4000 cm-1. Spectra were collected for fingernails before and after spiking with cocaine hydrochloride. Measurements were taken at one week and up to six weeks. Principal component analysis (PCA) revealed distinct clusters within the PC scores of cocaine-spiked versus unspiked fingernails. Findings showed that ATR-FT-IR spectroscopy could characterize fingernails based on intrinsic components and identify the presence or absence of cocaine within them.
The study of keratinized matrices, such as hair and fingernails, has become important in clinical and forensic toxicology because of their potential for drug accumulation. Keratinized matrices offer a greater window of detection for drugs over traditional body fluids such as blood and urine (1–3). Hair analysis has been used for detecting historic drug use in a range of forensic applications including suspicious deaths and drug-facilitated crimes (4,5). However, hair analysis is often subject to contamination by microorganisms and environmental dust or smoke (6–8).
Fingernail sampling is non-invasive, and it does not require medical personnel for its collection, making it an ideal alternative matrix to hair. Fingernail sampling offers a further advantage in detecting a wide range of analytes because of the slow growth rate of fingernails and their higher drug accumulation rate. Chemical analysis of nails has been used for a variety of purposes such as forensic investigations, occupational, and environmental exposure (9–11). Fingernail analysis has also been used to diagnose various diseases and monitor specific medicines (antidepressants and benzodiazepines) and drugs of abuse (amphetamines, cocaine, and opiates) (12–16).
Many studies have applied analytical techniques for measurement of drugs of abuse in fingernails. These studies have focused on classical analytical techniques such as gas chromatography–mass spectrometry (GC–MS) and liquid chromatography–mass spectrometry (LC–MS), and were semi-quantitative in nature (17,18). However, both GC–MS and LC–MS required expensive and extensive extraction of the analytes prior to analysis. Considering these concerns, we propose a unique method for cocaine analysis based on ATR-FT-IR spectroscopy, which is cost-effective, rapid, and requires minimum sample preparation. ATR-FT-IR spectroscopy has become a crucial technique for forensic researchers and investigators to assess trace evidence alongside drugs, inks, explosives, biological fluids, and cosmetics (19–23).
Cocaine is Europe’s most used illicit stimulant and remains in high supply and demand (24). Cocaine addicts must be monitored in a therapeutic or forensic/legal context, necessitating the use of multi-analyte methods that may identify several substances in a single run. This study presents a simple and sensitive ATR-FT-IR method in conjunction with chemometric tools for the detection of constituents and drugs in fingernails.
Experimental
This work comprised ATR-FT-IR spectroscopic analysis of 19 sets (11 males and eight females) of fingernails from adults of different ethnicities, each with various diets, medication or drug use, and lifestyles (Table I). Fingernail clippings were collected alongside a checklist regarding their medication use and lifestyle. No intervention was made by the researchers in terms of collecting the fingernail clippings, considering the procedure as an everyday activity. Ethical approval was granted from the two institutions that held the study: Liverpool John Moores University in the UK (PBS/2021-22/04) and the Lebanese University in Lebanon (2022-0104).
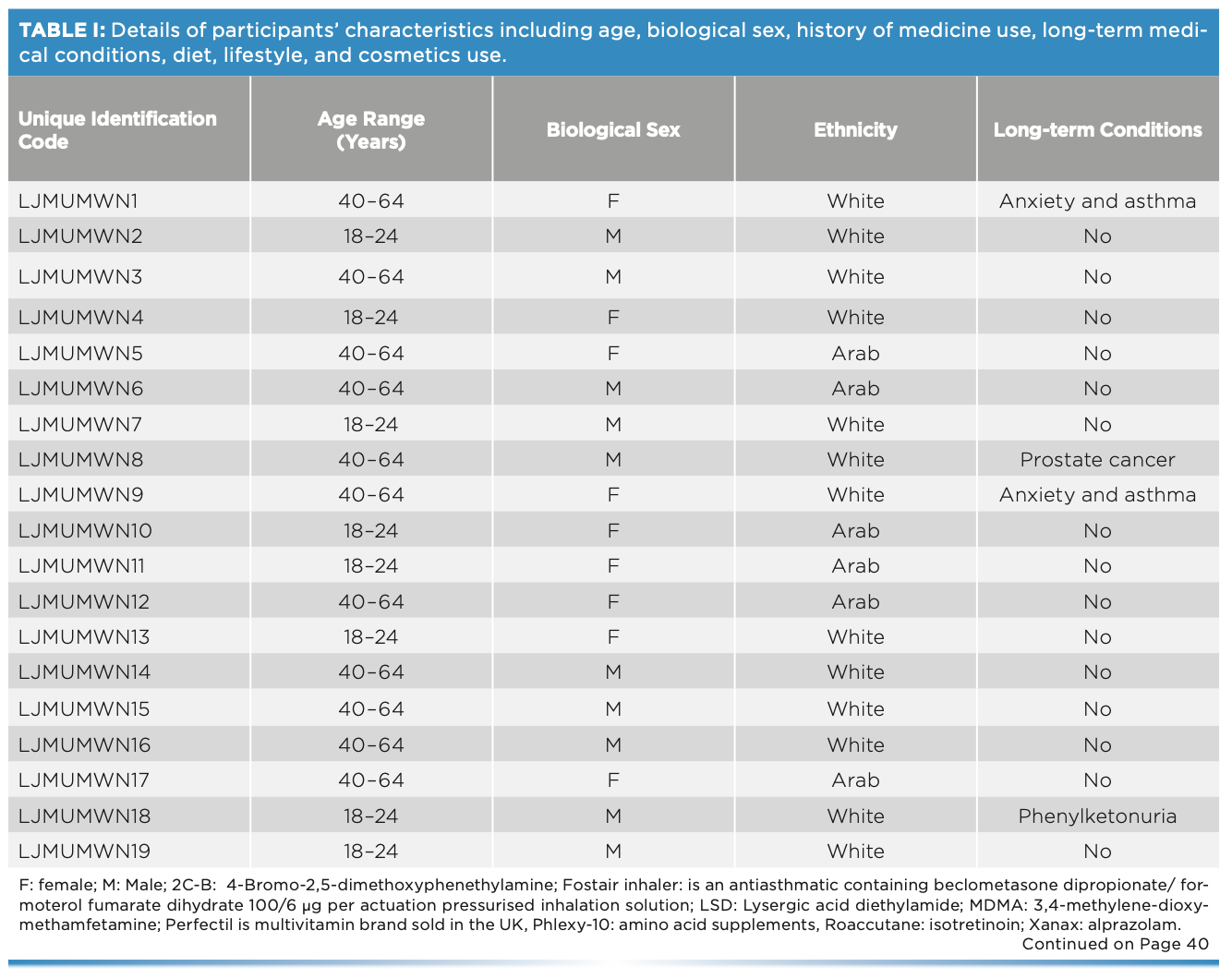
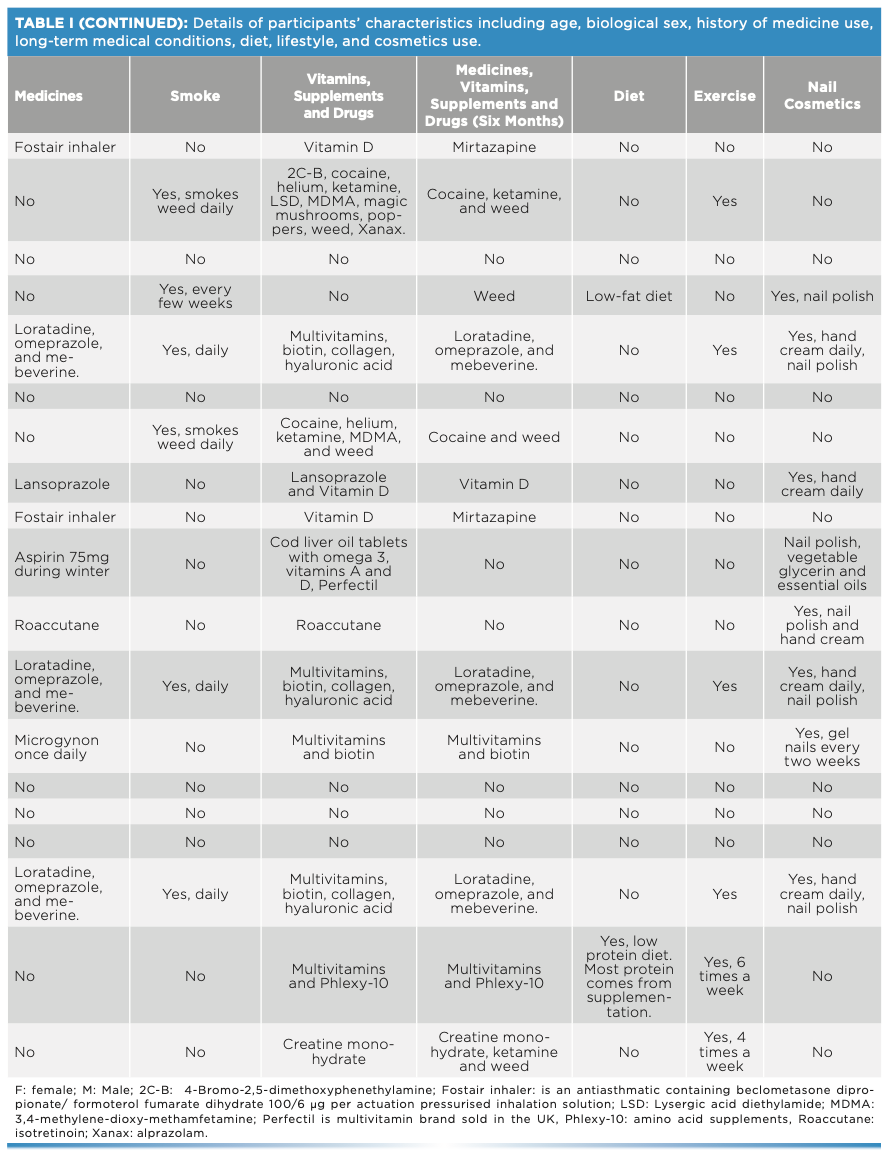
One fingernail set was placed in a glass vial and spiked with 50 mg of cocaine hydrochloride. The vial was vortexed for 2 min, coating the fingernails in the cocaine. Measurements were taken at weekly intervals over a six-week period. Spectral measurements were made using the Agilent 4500 ATR-FT-IR spectrometer over the wavenumber range of 650–4000 cm-1 and spectral resolution of 4 cm-1. Three spectra were collected per fingernail such that each spectrum was the sum of 64 scans.
Spectra were exported into Matlab 2019a for spectral interpretation, spectral quality evaluation, and analysis. For spectral interpretation, the absorption bands of cocaine hydrochloride and fingernails were evaluated. The number of peaks (N), wavenumber range, maximum peak intensity and position, and signal-to-noise ratio (S/N) were all considered while evaluating spectral quality (Table II). Principal component analysis (PCA) was applied to the nail spectra to visualize the patterns among the different participants’ fingernails, and the patterns among cocaine-spiked and unspiked fingernails. For PCA analysis, PC scores and loading plots were evaluated. PC scores demonstrated the patterns of clusters among the different fingernails, and PC loadings justified the importance of the clusters. PC loading plots showed the key functional groups that expressed the highest variance among the data. In this respect, PC1 expressed the highest variance, PC2 the second highest variance, and PC3 the third highest variance. For accuracy and precision of the PCA model, clusters were inspected for type I and type II errors. Type I errors were encountered when scores of the same fingernail set were not grouped together, whereas type II errors were seen when a score of one fingernail set was grouped with a different set.
Results and Discussion
The use of ATR-FT-IR for fingernail inspection showed many advantages in forensic analysis. First, the collection procedure was non-invasive and non-destructive, and it needed a small sample size (1–3 mm), requiring minimal intervention from the researcher.
Second, FT-IR spectra provided unique identifiable features relating to endogenous constituents within nails. These identifiable features can be used to understand individuals’ diets, disease states, drug/medicine usage, and their lifestyle characteristics. Third, previous studies have shown that spectral data of fingernails could highlight the presence of some physical and psychological conditions. For example, conditions such as anxiety, depression and diabetes affect the fingernail’s keratin, and this is observed through the nail’s appearance (12). Individuals who have chronic depression or systematic diseases are likely to possess discolored nails, fingernail plate alterations, or brittle fingernails (12). Disfiguration to the nail plate is often the consequence of altered trace element levels during chronic depression. Research has highlighted a strong association between decreased levels of nail constituents and depression (12). By optimizing the method for a portable device, it could be used on-site at airports, police stations, hospitals, and crime scenes.
This study aimed to complement previous studies by exploring the effects of different diets and lifestyles on the IR spectra of fingernails. Moreover, it assessed deposition of cocaine in nails upon chronic exposure over a period of six weeks. In turn, this enabled discrimination of chronic dealing with cocaine that has been of interest in forensic investigations. In this respect, it has been important to distinguish individuals suspected of possessing cocaine for a “one-use” from those who possess drugs chronically with intent to supply.
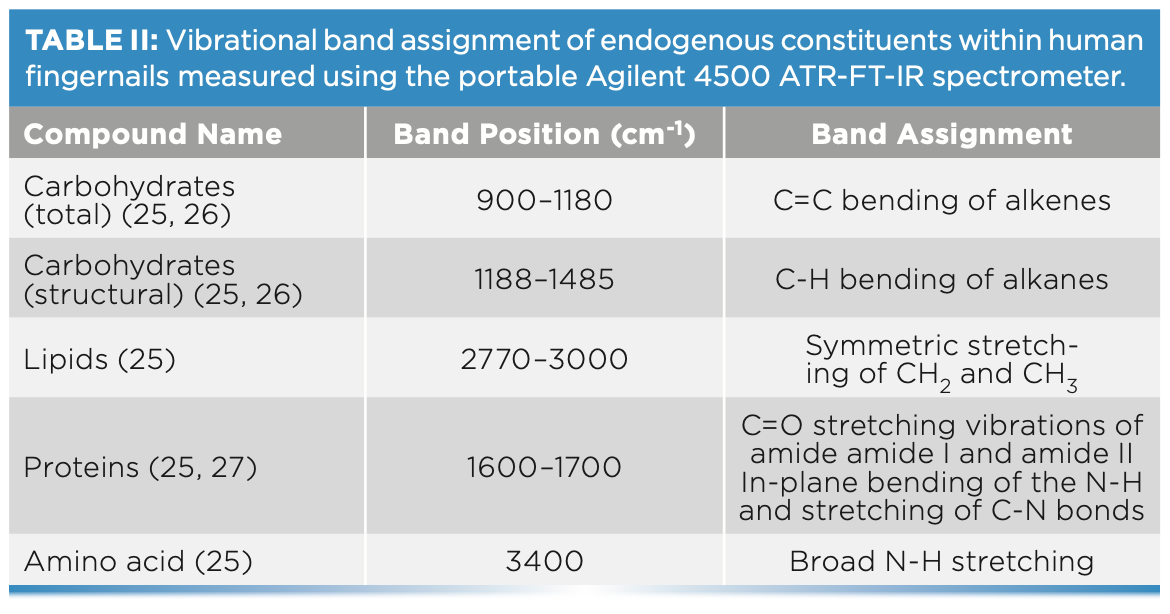
FT-IR Band Assignments of Fingernails
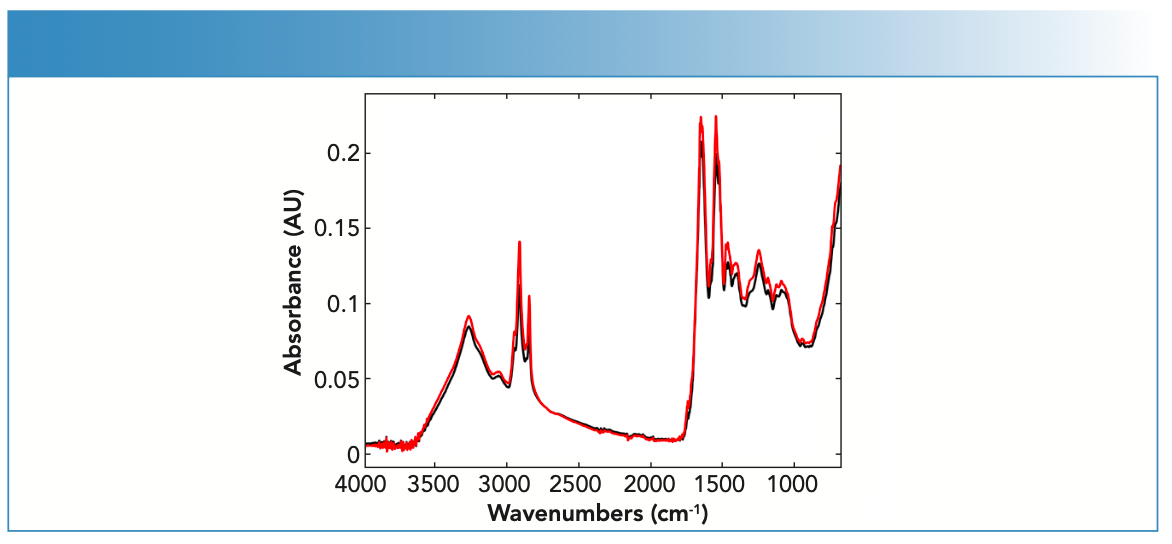
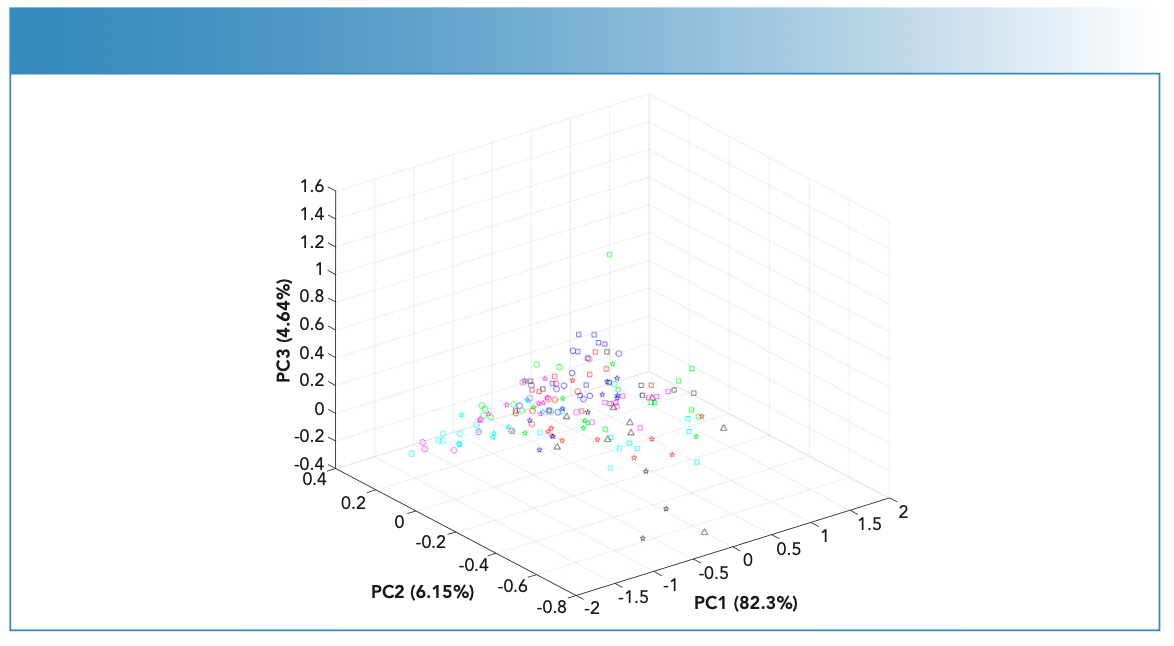
FT-IR spectra of the fingernail sets (n = 19) showed bands corresponding to the absorbances of key endogenous constituents usually present in fingernails (Figure 1). These constituents included proteins, lipids, carbohydrates, and amino acids (25). The identified bands were assigned vibrational bands (Table II).
FIGURE 1: Raw IR spectra of fingernail subject (nail set) #1 (black) and subject (nail set) #9 (red) measured using the portable ATR-FT-IR spectrometer.
Spectral Quality
Table III shows the IR spectra quality of the fingernail sets (n = 19). In this respect, fingernail sets showed strong (n = 7) or medium (n = 12) IR activity. Considering the four evaluation parameters, the number of bands (N) ranged from 19–25 bands for fingernail sets with strong IR activity, and from 16–25 bands for fingernail sets with medium IR activity. Having more IR bands allowed better discrimination between fingernails’ sets. This is especially advantageous in early disease diagnosis, where pattern recognition between healthy and diseased fingernails can be established (12). Despite differences in the number of bands, all fingernail sets shared the same maximum band position (between 1630–1650 cm-1) that corresponded to the fingernails’ proteins. The absorbance intensity of the protein band was in the range of 0.02–0.25 absorbance units. It is worth noting that the absorbance of 0.02 was seen for an individual with anxiety (LJMUWN1), confirming the findings of Cashman and Sloan’s 2010 study, which suggested that psychological conditions can affect a nail’s keratin (12). In addition, S/N varied between nails and was seen in the range of 50.5–130 for fingernails with strong IR activity, and in the range of 5.4–43.3 for fingernails with medium IR activity. Low S/N was seen for fingernails with high moisture content; this could be attributed to the individuals’ diets.
Fingernail Classification
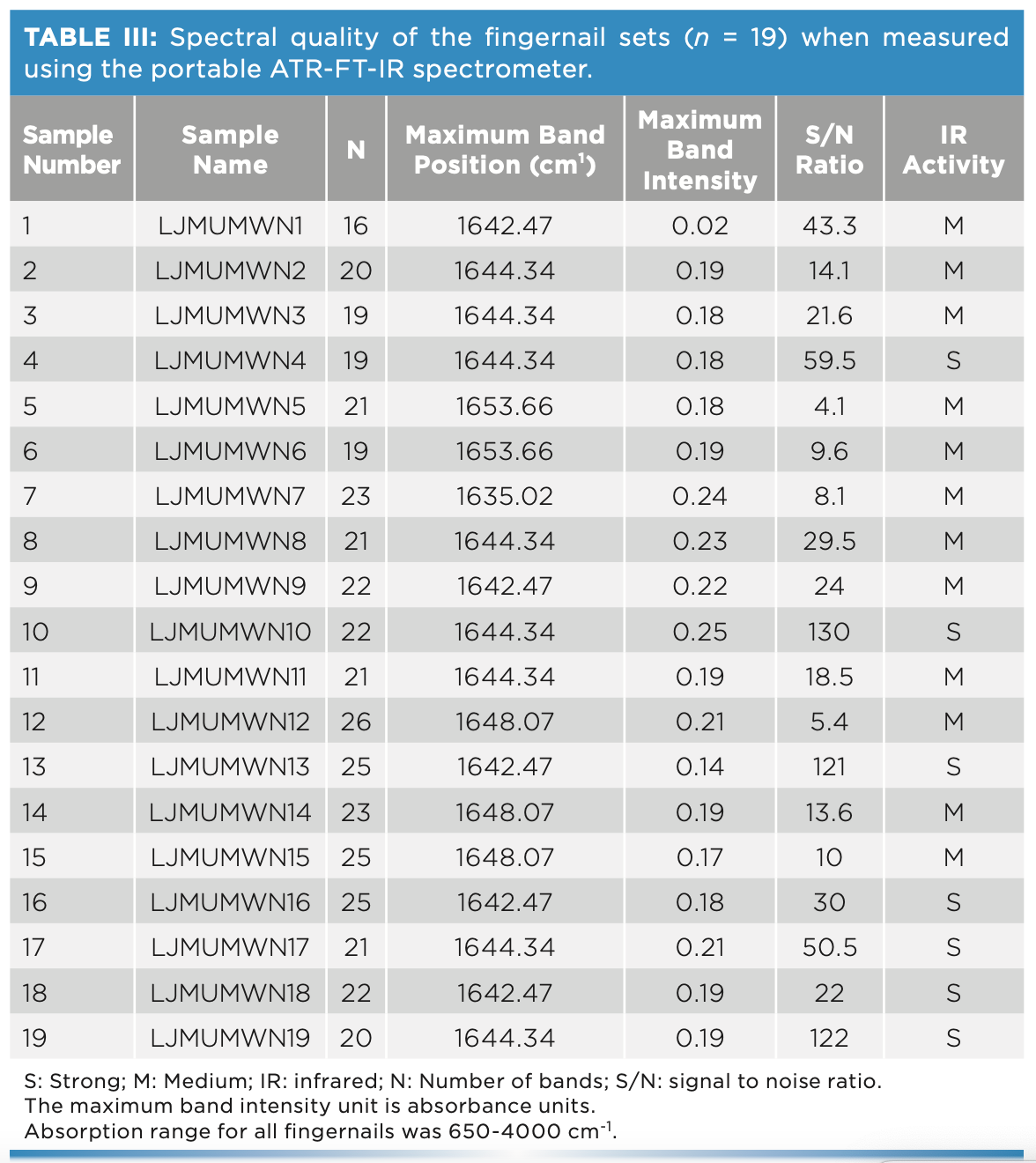
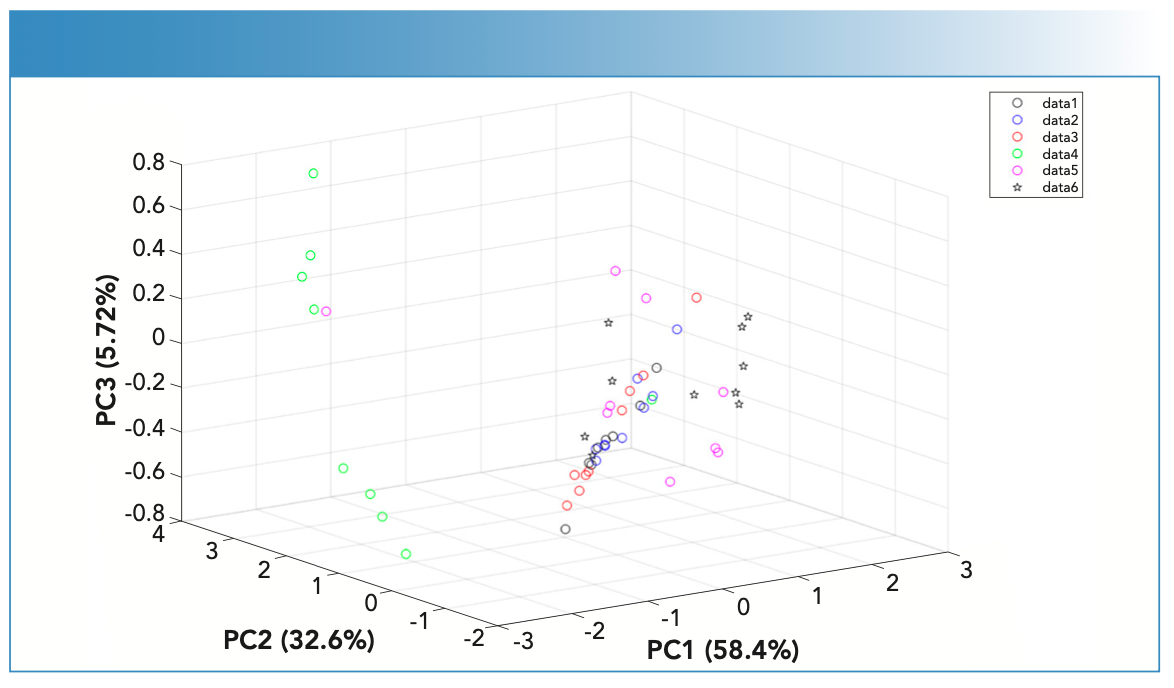
PCA analysis was often applied to large data sets to reduce dimensionality of data and to increase interpretability without information loss (Figure 2). During this analysis, spectral data was classified into the subspaces of PC scores and PC loading plots. The PC scores showed patterns of clusters among the different fingernails; whereas the PC loadings indicated which absorbances were important for each PC loading. When applied to the 19 sets of fingernails, the first three PC scores captured 93.1% of the variance but showed overlap among the fingernail scores apart from two fingernail sets. The latter two sets (5 and 6) were from individuals of Arab ethnicity; this finding demonstrated the ability of IR to classify participants’ demographic characteristics. This would be beneficial in disease management where personalized therapy is required.
FIGURE 2: PC scores plot of the FT-IR spectra of the fingernail sets (n = 19) measured using the portable ATR-FT-IR spectrometer. Nail set one (black circles), nail set two (blue circles), nail set three (red circles), nail set four (green circles), nail set five (magenta circles), nail set six (cyan circles), nail set seven (black squares), nail set eight (blue squares), nail set nine (red squares), nail set ten (green squares), nail set eleven (magenta squares), nail set twelve (cyan squares), nail set thirteen (black pentagons), nail set fourteen (blue pentagons), nail set sixteen (red pentagons), nail set seventeen (magenta pentagons), nail set eight (cyan pentagons), nail set nineteen (black triangles).
Detection of Cocaine in Nails
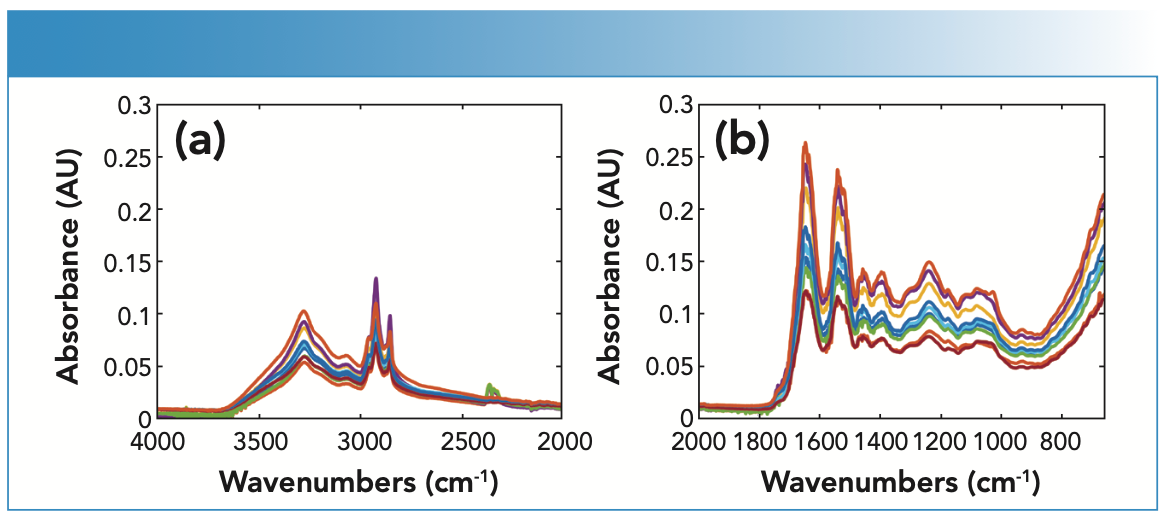
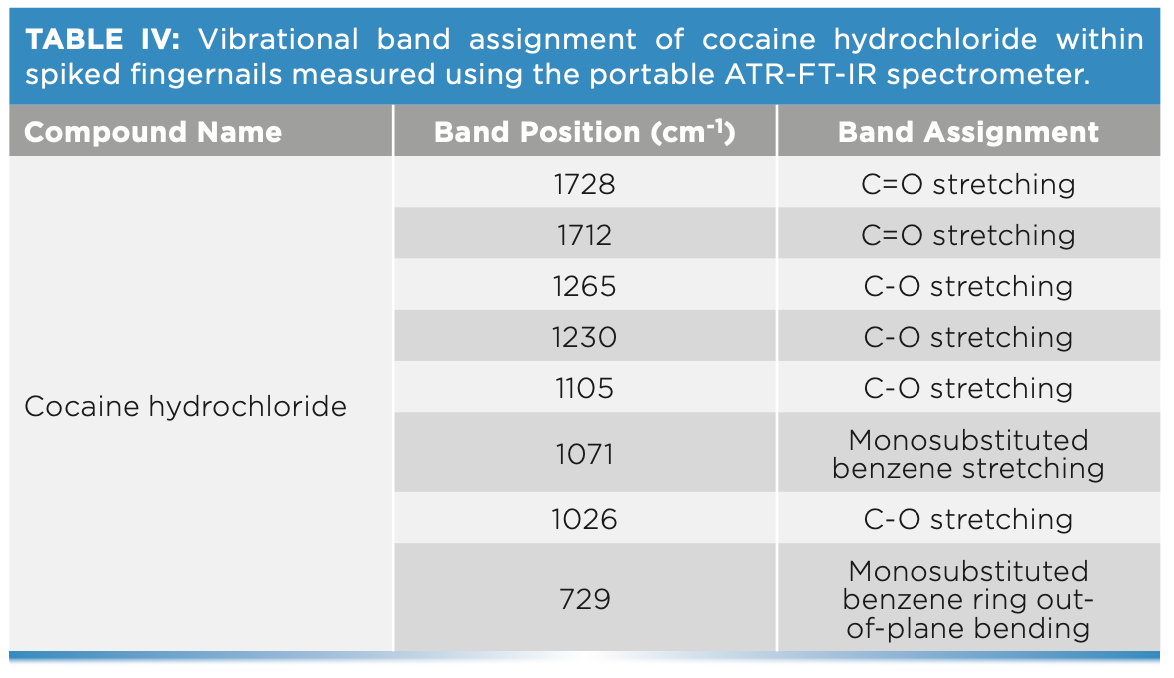
The ability of ATR-FT-IR to detect cocaine has been evaluated through the analysis of fingernail clippings spiked with cocaine hydrochloride for a period of six weeks (Figure 3). Table IV shows the main bands attributed to cocaine hydrochloride that were featured in the spectra of fingernails. The key band attributed to the benzene ring stretching at 1072 cm-1 was featured in the nail spectra in weeks 1–6. This further confirmed that fingernails act as a drug depot that is accumulating drugs over time. This latter finding supports previous literature that identified historic drug use through the analysis of nails as an alternative matrix. A greater window of detection could be attributed to how the drug is deposited into the fingernail through the blood supply. In this sense, drugs are deposited by diffusion into the germinal matrix and nail bed, which grows at a rate of 3.47 mm per month (1). As the fingernail grows, the drug is deposited horizontally and vertically into the fingernail, which can explain drug detection up to six months after consumption.
FIGURE 3: (a) Raw FT-IR spectra of fingernails, and (b) spiked with cocaine for a period of six weeks measured using the portable ATR-FT-IR spectrometer.
Cocaine has a high affinity to proteins, resulting in cocaine–protein interactions (28). These interactions are reliant on the concentration of the cocaine one consumed or was exposed to (28). Therefore, individuals who are chronically exposed to cocaine are more likely to have higher concentrations of cocaine within their fingernails and more cocaine–protein interactions. As previously mentioned, fingernails are composed of a laminated keratinized structure of alpha keratin (29). Keratins are a group of structural fibrous proteins that are often found in ectodermal tissues, hair, and nails (29). Unlike other structural proteins, such as collagen and elastin, which are found in bones and teeth, keratin has sulphur-rich amino acids, cysteine, and disulfide links (30). Therefore, cocaine has a high affinity for proteins within the fingernail and is easily deposited.
To visualize patterns among the spectra of the cocaine-spiked fingernails’ spectra over the six-week period, PCA models were applied. The first three PCs contributed to 96.7% of the variance among the data (Figure 4). PC scores indicated three distinct clusters, yet an overlap was found within and between the cluster. Hence, the first cluster showed the highest variance among the data and contained scores corresponding to weeks five and six. This indicated that cocaine deposited over a longer duration of time; this was further confirmed by the PC1 loading that showed bands corresponding to cocaine. Cluster two showed the second highest variances among the data and contained scores of weeks one and three, in addition to one score for week four, four scores for week five, and three scores for week six. This showed type II errors where the scores of weeks four, five and six were clustered with weeks one and three. Cluster three showed scores with the least variance and corresponded to week four with one score of week five. This could be attributed to the change resulting from the cocaine interacting with the fingernail’s proteins; however, further research is required for confirming such interaction. Nonetheless, the pattern of clustering has shown that PCA was successful in monitoring the depot.
FIGURE 4: PC scores plot of fingernail clippings spiked with cocaine for one to six weeks using the portable ATR-FT-IR spectrometer. Week one of spiking (black circles), week two of spiking (blue circles), week three of spiking (red circles) week four of spiking (green circles), week five of spiking (magenta circles), and week six of spiking (black pentagons).
Conclusion
ATR-FT-IR spectroscopy offered a rapid and portable technique for the detection of endogenous constituents and cocaine within fingernails. The results showed that FT-IR spectra of fingernails had bands corresponding to carbohydrates at 926 and 1045 cm-1, lipid bands at 2958, 2921, and 2851 cm-1, and protein bands at 1644 and 1536 cm-1. Analysis of cocaine-spiked fingernails demonstrated cocaine bands at 978 cm-1 (monosubstituted out-of-plane bending vibration), 1010 cm-1 (CO stretching), 1027 cm-1 (monosubstituted benzene ring stretching), 1055 cm-1 (CO and CN stretching), 1071 cm-1 (monosubstituted benzene ring stretching), and 1105 cm-1 (CO stretching). The results also demonstrated that the IR spectra of the fingernail sets indicated individuals’ characteristics and disease statuses that influenced the protein constituents featured in the IR spectra. Furthermore, the length of exposure to cocaine influenced the depositing of the drug within the fingernail and increased the likelihood of cocaine-protein interactions. Subsequently, chronic exposure increased the probability of drug detection, providing useful information for courts when investigating charges of possession with intent to supply. In the future, a multivariate quantitative approach (principal component regression) would be ideal to track changes in cocaine concentration within fingernails overtime.
References
(1) Cappelle, D.; Yegles, M.; Neels, H.; van Nuijs, A. L. N.; De Doncker, M.; Maudens, K.; Covaci, A.; Crunelle, C. L. Nail Analysis for the Detection of Drugs of Abuse and Pharmaceuticals: A Review. Forensic Toxicol. 2015, 33 (1), 12–36. DOI: 10.1007/s11419-014-0258-1
(2) Pragst, F.; Balikova, M. L. State of the Art in Hair Analysis for Detection of Drug and Alcohol Abuse. Clin. Chim. Acta. 2006, 370 (1–2), 17–49. DOI: 10.1016/j.cca.2006.02.019
(3) Verstraete, A. G. Detection Times of Drugs of Abuse in Blood, Urine and Oral Fluid. Ther. Drug Monit. 2004, 26 (2), 200–205. DOI: 10.1097/00007691-200404000-00020
(4) Uhl, M. Determination of Drugs in Hair Using GC/MS/MS. Forensic Sci. Int. 1997, 84, 281–294. DOI: 10.1016/s0379-0738(96)02072-5
(5) Kronstrand, R.; Nystrom, I.; Josefsson, M.; Hodgins, S. Segmental Ion Spray LC-MS-MS Analysis of Benzodiazepines in Hair of Psychiatric Patients. J. Anal. Toxicol. 2002, 26, 479–484. DOI: 10.1093/jat/26.7.479
(6) Romano, G.; Barbera, N.; Lombardo, I. Hair Testing for Drugs of Abuse: Evaluation of External Cocaine Contamination and Risk of False Positives. Forensic Sci Int. 2001, 123, 119–129. DOI: 10.1016/s0379-0738(01)00539-4
(7) Auwärter, V.; Wohlfarth, A.; Traber, J.; Thieme, D.; Weinmann, W. Hair Analysis for Delta9-tetrahydrocannabinolic Acid A--New Insights Into the Mechanism of Drug Incorporation of Cannabinoids Into Hair. Forensic Sci Int. 2010, 196 (1–3), 10–13. DOI: 10.1016/j.forsciint.2009.12.023
(8) Romano, G.; Barbera, N.; Spadaro, G.; Valenti, V. Determination of Drugs of Abuse in Hair: Evaluation of External Heroin Contamination and Risk of False Positives. Forensic Sci Int. 2003, 131 (2–3), 98–102. DOI: 10.1016/s0379-0738(02)00413-9
(9) Samanta, G.; Sharma, R.; Roychowdhury, T.; Chakraborti, D. Arsensic and other Elements in Hair, Nails, and Skin-scales of Arsenic Victims in West Bengal, India. Sci. Total Environ. 2004, 326 (1–2), 33–47. DOI: 10.1016/j.scitotenv.2003.12.006
(10) Moretti, M.; Andrello, L.; Visonà, S.; Vignali, C.; Groppi, A.; Freni, F.; Osculati, A.; Tajana, L.; Morini, L. Evaluation of Benzodiazepines and Zolpidem in Nails and Their Stability After Prolonged Exposure to Chlorinated Water. J. Pharm. Biomed. Anal. 2018, 152, 137–142. DOI: https://doi.org/10.1016/j.jpba.2018.01.051
(11) Wilhelm, M.; Hafner, D.; Lombeck, I.; Ohnesorge, F. K. Monitoring of Cadmium, Copper, Lead and Zinc Status in Young Children Using Toenails: Comparison with Scalp Hair. Sci. Total. Environ. 1991, 103 (2-3), 199–207. DOI: 10.1016/0048-9697(91)90145-5
(12) Cashman, M.; Sloan, S. Nutrition and Nail Disease. Clin. Dermatol. 2010, 28 (4), 420–425. DOI: 10.1016/j.clindermatol.2010.03.037
(13) Palmeri, A.; Pichini, S.; Pacifici, R.; Zuccaro, P.; Lopez, A. Drugs in Nails: Physiology, Pharmacokinetics and Forensic Toxicology. Clin. Pharmacokinet. 2000, 38 (2), 95–110. DOI: 10.2165/00003088-200038020-00001
(14) Garside, D. Drugs-of-Abuse in Nails. In Drug Testing in Alternate Biological Specimens, Jenkins, A. J. Ed. Humana Press, 2008, pp. 43–65.
(15) Morini, L.; Colucci, M.; Ruberto, M.; Groppi, A. Determination of Ethyl Glucuronide in Nail by Liq- uid Chromatography Tandem Mass Spectrometry as a Potential New Biomarker for Chronic Alcohol Abuse and Binge Drinking Behavior. Anal. Bioanal. Chem. 2012, 402 (5), 1865–1870. DOI: 10.1007/s00216-011-5609-8
(16) Pufal, E.; Sykutera, M.; Piotrowski, P. Development of a Method for Determining Antidepressant Drugs in Nails. Arch Med Sadowej Kryminol. 2008, 58 (4), 167–170.
(17) Garside, D.; Ropero-Miller, J. D; Goldberger, B. A.; Hamilton, W. F.; Maples, W. R. Identification of Cocaine Analytes in Fingernail and Toenail Specimen. Anal. Toxicol. 1998, 43 (5), 974–979.
(18) Cingolani, M.; Scavella, S.; Mencarelli, R.; Mirtella, D.; Froldi, R.; Rodriguez, D. Simultaneous Detection and Quantitation of Morphine, 6-Acetylmorphine, and Cocaine in Toenails: Comparison with Hair Analysis. J. Anal. Toxicol. 2004, 28 (2), 128–131. DOI: 10.1093/jat/28.2.128
(19) Marcelo, M. C. A.; Mariotti, K. C.; Ferräo, M. F.; Ortiz, P. S. Profiling Cocaine by ATR-FTIR. Forensic Sci. Int. 2015, 246, 65–71. DOI: 10.1016/j.forsciint.2014.11.011
(20) Mohamad Asri, M. N.; Mat Desa, W. N. S.; Ismail, D. Combined Principal Component Analysis (PCA) and Hierarchical Cluster Analysis (HCA): An Efficient Chemometric Approach in Aged Gel Inks Discrimination. Aust. J. Forensic Sci. 2018, 52 (9), 1–22. DOI: 10.1080/00450618.2018.1466913
(21) Mou, Y.; Rabalais, J. W. Detection and Identification of Explosive Particles in Fingernails Using Total Reflection-Fourier Transform Infrared Spectromicroscopy. J. Forensic Sci. 2009, 54 (4), 846–850. DOI: 10.1111/j.1556-4029.2009.01060.x
(22) Sharma, S.; Singh, R. Detection and Discrimination of Seminal Fluid Using Attenuated Total Reflectance-Fourier Transform Fnfrared (ATR FT-IR) Spectroscopy Combined with Chemometrics. Int. J. Legal Med. 2020, 134, 411-432. DOI: 10.1007/s00414-019-02222-x
(23) Chophi, R.; Sharma, S.; Singh, R. Discrimination of Nail Polish Using Attenuated Total Reflectance Infrared Spectroscopy and Chemometrics. Aust. J. Forensic Sci. 2020, 53 (3), 1–12. DOI: 10.1080/00450618.2020.1713212
(24) Publication Office of the European Union. European Drug Report 2017: Trends and Developments, 2017. https://www.emcdda.europa.eu/ publications/edr/trends-developments/2017_en (accessed 2023-07- 03).
(25) Gniadecka, M.; Nielsen, O.; Christensen, D.; Wulf, H. Structure of Water, Proteins, and Lipids in Intact Human Skin, Hair, and Nail. J. Invest. Dermatol. 1998, 110 (4), 393–398. DOI: 10.1046/j.15231747.1998.00146.x
(26) Yari, M.; Valizadeh, R.; Nnaserian, A.; Jonker, A.; Yu, P. Carbohydrate and Lipid Spectroscopic Molecular Structures of Different Alfalfa Hay and Their Relationship with Nutrient Availability in Ruminants. Asian-Australas J. Anim. Sci. 2017, 30 (11), 1575–1589. DOI: 10.5713/ajas.16.0756
(27) B. Singh, Infrared Analysis of Peptides and Proteins; American Chemical Society, 2000.
(28) Assi, S.; Arafat, B.; Lawson-Wood, K.; Robertson, I. Authentication of Antibiotics Using Portable Near-Infrared Spectroscopy and Multivariate Data Analysis. Appl. Spectrosc. 2020, 75 (4), 434–444. DOI: 10.1177/0003702820958081
(29) Heard, K.; Palmer, R.; Zahniser, N. Mechanisms of Acute Cocaine Toxicity. Open Pharmacol. J. 2008, 2 (9), 70–78. DOI: 10.2174/1874143600802010070
(30) De Berker, D.; Wojnarowska, F.; Sviland, L.; Westgate, G.; Dawber, R.; Leigh, I. Keratin Expression in the Normal Nail Unit: Markers of Regional Differentiation. Br. J. Dermatol. 2000, 142 (1), 89–96. DOI: 10.1046/j.1365-2133.2000.03246.x
(31) Baraldi, A.; Jones, S.; Guesné, S.; Traynor, M.; McAuley, W.; Brown, M.; Murdan, S. Human Nail Plate Modifications Induced by Onychomycosis: Implications for Topical Therapy. Pharmacol. Res. 2015, 32, 1626–1633.
Megan Wilson, Jason Birkett, Iftikhar Khan, and Sulaf Assi are with the School of Pharmacy and Biomolecular Sciences at Liverpool John Moores University, in Liverpool, United Kingdom. Ismail Abbas is with the Faculty of Science at Lebanese University, in Beirut, Lebanon. Leung Tang is with the Chemical Analysis Group at Agilent Technologies, in Cheadle, United Kingdom. Dhiya Al-Jumeily is with the School of Computer Sciences and Mathematics at Liverpool John Moores University, in Liverpool, United Kingdom, Direct correspondence to: m.wilson3@2019.ljmu.ac.uk ●

Measurement of Ammonia Leakage by TDLAS in Mid-Infrared Combined with an EMD-SG Filter Method
April 9th 2024In this article, tunable diode laser absorption spectroscopy (TDLAS) is used to measure ammonia leakage, where a new denoising method combining empirical mode decomposition with the Savitzky-Golay smoothing algorithm (EMD-SG) is proposed to improve the signal-to-noise ratio (SNR) of absorbance signals.
Childhood Obesity Serum Samples Analyzed Using Fourier Transform Infrared Spectroscopy
April 9th 2024A group of scientists led by Zozan Guleken of the Gaziantep University of Science and Technology in Gaziantep, Turkey looked into the blood serum composition of childhood obesity (CO) tissues using Fourier transform infrared (FT-IR) spectroscopy.