Ion Mobility Spectrometry: Solo or Coupled to Mass Spectrometry
A review of the basics of ion mobility spectrometry and field-asymmetric ion mobility spectrometry (FAIMS), plus how to couple FAIMS to mass spectrometry

Ion mobility spectrometry (IMS) is a method for the separation of ions, which, as an analytical technique, has been applied for environmental monitoring, rapid security screening, and characterization of high mass biomolecular ions. In this column, we review the basics of IMS, describe field asymmetric ion mobility spectrometry (FAIMS), and discuss coupling FAIMS with mass spectrometry in an instrument that produces higher-dimensional data useful for the analysis of complex mixtures.
At its core, mass spectrometry (MS) involves the measurement of the mass of an ion. In various instruments, we use electric and magnetic fields to do so, because the mass of the ion appears in the equations that describe the forces exerted on the ion by those fields. The charge on the ions also appears in the equations — it is the handle that allows the manipulation of the ions, but it can be a single or a multiple of the basic charge unit. Measuring the masses of neutral molecules is more difficult because we cannot rely on the charge as a handle. And in contrast to mass spectrometers that can measure the mass of a single ion in the right circumstances, when we deal with neutral molecules, we usually observe a differential behavior that requires a collection of many molecules for discernment. As an example, we can determine the masses of larger neutral molecules by classic methods such as planar electrophoresis or gel permeation chromatography; these are classified as separation rather than spectroscopic methods, but mass is one of the parameters measured. Moving to spectroscopy, masses of individual molecules can sometimes be determined by atomic force microscopy or more commonly estimated by scanning transmission electron microscopy. The chromatographic methods operate in an environment quite different from the typical vacuum of the mass spectrometer. In chromatography, we want the molecules to interact with the chromatographic medium. In removing gas molecules by establishing a vacuum, we ensure that collisions of moving ions with gas molecules don't mask the differential interaction with the fields in proportion to the ions' mass-to-charge ratios. We measure that isolated behavior, separate the ions based on it, and produce the mass spectrum by comparison with mass standards.
Ion Mobility Spectrometry
Ion mobility spectrometry (IMS) does not take place in a vacuum; it is different from other separation methods in that it does not rely on the interactions of the moving particles with a fixed medium. In IMS, the medium is a drift gas flowing in the opposite direction to that of the ions.
IMS, like MS, is a means of separating ions of different characteristics. Before starting with a fundamental review of IMS, let's think for a minute about the ions. An ion has a mass, and for any particular polyatomic ion, that mass is based on exactly which atomic isotopes are present in that particular ion. For a population of ions of known formula, we know what the exact mass should be and we know what the isotopic distribution should be. An ion has a charge, either a single or a multiple charge of either polarity; the distribution of the charge states is of interest. For much of the past hundred years of MS, the vast majority of measured ions were singly charged, whether positive or negative. We learned how to ionize larger molecules, and these could often be formed in multiple charged states. But the measured mass was still the sum of the masses of the individual atoms. Of course, an ion has a structure; for ions formed from organic compounds of a few hundred or a thousand daltons in mass, that structure is the bond-by-bond linkage between atoms. We interpret the fragmentations evident in the mass spectrum to discern that structure, and we could deduce some limited three-dimensional information. But for the purposes of the measurement, the ion was still a point.
But a larger ion has definite three-dimensional structure. It has a size and a shape, and in fact, it can have several different sizes and shapes that do not necessarily have to be in equilibrium with each other. The sizes and shapes an ion assumes depend on its internal energy, its state of charge, and the surrounding environment — whether that environment is a solution, a gas (at varying pressures or temperatures), or a vacuum (as in a mass spectrometer). As an example, a protein molecule or ion can be in folded or unfolded states or in intermediate conformations that are based on intermolecular factors such as hydrogen bonding, the number and distribution of charges, and also interaction with its environment. IMS separates ions based on their differential mobilities as the ions travel through a drift gas in a tube exhibiting a constant electric field gradient. The separation of the ions is based on a simple concept — ions of different sizes and shapes (and different charge states too) will travel at different velocities. Ions of different sizes and shapes may, but are not required to, have different masses. Conversely, ions of the same mass may, but are not required to, have the same size and shape. In IMS, repeated collisions of the ions with the drift gas are integral to the measurement. The ion mobility can be measured at different pressures, including atmospheric pressure and reduced pressures. But the IMS instrument does not operate under vacuum as does a mass spectrometer.
IMS was originally known as plasma chromatography, and MS was used to identify some of the relevant reactant species (1,2). Ions are formed in any of a variety of methods, and a discrete pulse is used to inject the ions into the tube at one end (analogous to the ionization source of a mass spectrometer). The modest field gradient in the tube accelerates the ion. However, because of the drift gas moving in a direction opposite to that of the ion acceleration, the moving ion soon collides with a gas molecule, which impedes its travel. After collision, the ion again accelerates, but it soon collides again. This sequence is repeated by all of the ions in the injected population. It is not the velocity of an individual ion that is measured, but rather the velocity of the population of ions. Ions of the same size and shape, resulting in the same ion mobility, will exhibit the same constant ion velocity over the distance of the flight tube and will arrive at the detector at the same time. Ions with different sizes and shapes, because they have a different cross section for collision, will have different velocities, and therefore the ions are separated based on their size and shape.
The relevant equation in simple IMS is that for ion mobility, given as K. Under a fixed set of conditions the ion mobility is the ion's drift velocity (vD) divided by the applied electric field E:

The drift tube has a length L and what is measured is the drift time of the ion, tD. When U is the applied electric field gradient, the following equation applies:

Another relevant equation must incorporate the charge on the ion, its collision cross section s, and the temperature and pressure of the drift gas. All of these variables are brought together in the Mason equation (3), which appears in one form as
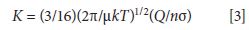
where K is again the ion mobility, σ is the collisional cross section of the ion, Q is the charge on the ion, n is a parameter related to the pressure and flow parameters of the drift gas, T is the temperature of the drift gas, µ is the reduced mass of the ion and the drift gas, and k is the Boltzmann constant. K increases with increased charge, smaller cross sections, and a lower resistance to flow (a decreased n). Note that there is no dependence of ion mobility on the applied field strength itself, at low enough values of U, and mass enters through the reduced mass term µ. The separation in IMS thus depends on mass and charge (which are examined directly in MS) but are convoluted with the collisional cross section (the ion's size, shape, and polarizability) and other parameters. IMS is a shape-to-charge-based separation method. A reduced mobility, K0, can be reported that factors in any changes in the drift gas pressure and temperature, as follows:
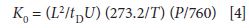
where L is the length of the drift tube, tD is the drift time, U is the applied electric field, and T and P are the experimental temperature and pressure, respectively.
What does the detector output for a simple IMS device look like? The instrument parameters are set to produce ion velocities of 1–10 m/s, which are much slower than the ion velocities through mass spectrometers. Given a common benchtop-scale instrument, the times for ions to pass through the instrument are in the 1–100 ms range. Time-of-flight (TOF) mass spectrometers are cycled about 103-fold more rapidly. However, just as many individual TOF mass spectra are averaged together to create a mass spectrum, many separate ion pulse measurements are averaged together to create an IMS output, which is a plot of measured ion current on the y-axis versus time on the x-axis. Small reactant ions or background ions arrive at low transit times; larger ions require a longer time to swim upstream against the drift gas and reach the detector. Figure 1 is a schematic of the simple output expected, showing peaks for reactant ions and product ions. The peaks are typically broad because of the variability in the collision processes. But just as in column chromatography, the transit time through the drift tube is characteristic. Selectivity can be built into the measurement with selection of the ionization process, following some of the same chemistry explored in detectors used for gas chromatography (GC) itself. Better separation resolution can be achieved by consideration of the basic parameters that affect the ion mobility. As in column liquid chromatography (LC) or GC, a theoretical plate height can be determined. Peak widths are determined by the width of the initial ion pulse and transport equations of ion motion that consider diffusion broadening. Hill and colleagues (4) explain these calculations in a 1990 review article and explore the limits on chromatographic resolution with a simple IMS device.
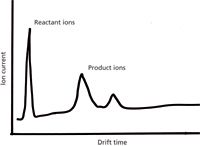
Figure 1: Simple ion mobility spectrum measurement of measured ion current (y-axis) versus travel time through the device (x-axis). Smaller reactant ions are well separated in drift time from larger product ions from the sample.
Field-Asymmetric Ion Mobility Spectrometry
At higher applied field strengths, the ion mobility exhibits a specific dependence on the field strength that is not apparent at lower fields. Therefore, ionic species that cannot be separated by IMS at the lower field strengths may become resolvable. In field-asymmetric IMS (FAIMS), the applied electric field is oscillated between a high and a low value. Ions that exhibit the same velocity at the lower field will be differentiated at the higher applied field. The potential resolving power is amplified by the fact that the change in ion mobility at higher fields can move in either direction or can change in different directions at different field strengths (see Figure 2), although most ionic species studied to date follow a unidirectional change. Verbeck and colleagues (5) have described resolution equations for this form of ionic separation. If the movement of ions through a region of alternating electric fields evokes the concept of a quadrupole mass filter used in MS, you have successfully predicted the next paragraph of this column.
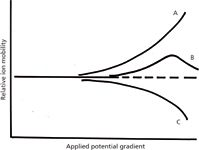
Figure 2: Differential ion mobilities as a function of applied field gradient. At low gradients, mobilities are independent of field, but at higher gradients, ions (A, B, and C) can exhibit several different behaviors that make the separation of similar species feasible.
In a development originally known as transverse field ion mobility mass spectrometry, the movement of the ionic species may not be through a drift tube of specified length, but between and through two parallel electrodes (Figure 3). The ions are injected in a pulse at one end of the device, and the drift gas carries the ions from the source to the detector, as contrasted with "opposing" the flow. The electric fields applied to the electrodes control which ions traverse the device to make it through to the detector. FAIMS devices operate at atmospheric pressure, and like IMS instruments, act as an ion separation device. Conditions are set (the combination of low field and high field potentials applied to the electrode, and drift gas velocity) so that certain ions get through, and others that intersect with the electrodes do not. As such, the device can serve as a specific detector (if the ion mobility can be directly and specifically related to the compound of interest), or as a selective source for another following device such as a mass spectrometer. If the characteristics of the targeted analyte of interest under a specified set of conditions are known, then the FAIMS instrument becomes a dedicated sensor of sorts. Miniaturized versions serve very specific applications in targeted compound detection, and a broad variety of instrumental designs have been explored.
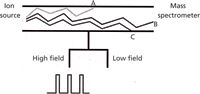
Figure 3: Modulation of gradients in an FAIMS device and modeled ion trajectories.
Of particular interest in the development of FAIMS are device designs that allow a direct coupling to a mass spectrometer (6–8). Because most FAIMS devices operate at atmospheric pressure, they can be linked to the source of appropriate mass spectrometers without major modifications. Any separation method used prior to, or as part of, the source of a mass spectrometer increases the analytical performance of the instrument by reducing sample overlap and by providing another "filter" for increased selectivity. The particular advantage of FAIMS is that it accomplishes separation based on a unique set of criteria, it can be used to separate isobaric and isomeric compounds, and it can be used with high mass biomolecular ions. In addition, and in contrast to IMS, FAIMS devices can provide a continuous current of selected ions to the mass spectrometer (or a changing population of ions, depending on the changes in the voltages used). The ions, with different mobilities, represent structures that are stable on the millisecond time scale of the experiment and in the absence of solvent. In fact, collisions with the drift gas may be used to coax the unfolding of large ions and reveal new conformations. Changing the ionization method that creates the ions in the first place can create highly charged expanded structures from what are usually more-compact ion species. Highly charged structures will expand as the dielectric constant of the medium changes. All of the different ion structures for the larger biomolecules can be compared with those structures predicted by calculation and the resultant calculated cross sections. However, when a FAIMS device is coupled with a mass spectrometer, we can also directly measure mass, charge, and reactivity information for the various ion shapes.
Commonly a FAIMS device is interfaced with an orthogonal TOF mass spectrometer (9). Remember that the drift times through the FAIMS device are on the order of milliseconds, and the TOF-MS system records a single mass spectrum within microseconds. Although multiple individual mass spectra are averaged together in TOF-MS, it is still possible to record multiple mass spectra within a time window of milliseconds as ion species pass through the FAIMS device. This approach is referred to as a "nested" measurement; it is no different conceptually from GC–MS or LC–MS measurements. Similarly, the data sets are complex, with a complete mass spectrum recorded for each of the FAIMS time windows. Families of ions with similar properties can be discerned in the data sets, but because the ion mobility is related to mass, the familial properties are those such as similar charge states or similar post-translational modifications.
However, there are advantages to the FAIMS-MS combination, derived from the fact that the species separated by FAIMS are, after all, ions. Ions can be excited by collision and dissociated almost at will in modern instruments, and this process can be induced to occur between (or before) analyses that separate them based on their shape-to-charge or mass-to-charge ratios. These changes are similar in concept and practice to those in MS-MS. In the FAIMS–MS application, conditions can be modulated between those that induce dissociation and those that do not, creating an additional dimension in the analysis. A fundamental limit on the dimensionality is related to the comparative time scales of the separation, dissociation, and mass analysis processes. Collision-induced dissociation, photo-induced dissociation, and electron-capture and electron-transfer dissociation processes can be used. All of these processes are fast relative to the FAIMS separation. The FAIMS separation is also fast compared to an LC separation; therefore an LC–FAIMS–MS device is not only feasible, but operational. As might be expected, the large-volume data set can be evaluated with the same correlative and data mining techniques used for other MS-based datasets. A comprehensive assessment of the data usually requires a far greater amount of time than the instrumental time required to acquire it.
Resolution in FAIMS has improved with the construction of tandem drift tube configurations (using ion funnels) that may use different drift gases and applied potential combinations. The time frame of separation is still on the order of milliseconds, but it is now subdivided into shorter periods. Are evanescent ion structures stable on the order of milliseconds as we tighten the time window? The answer seems to be yes — and we can resolve these structures with the higher dimensional data, including MS data, that we record. Once we know that there are multiple independent conformations, we can probe each of them individually or explore the conditions necessary to cause them to convert from one form to the other.
Conclusions
Ion mobility instruments span a range from miniaturized selective and field-portable devices to dedicated laboratory instruments applied to the current challenging problems in biomolecular analysis. Developments in MS-MS instrumentation parallel those in FAIMS–MS. A willingness to adopt, adapt, and apply has greatly accelerated the exploration of FAIMS–MS as a mature analytical method for biomolecule analysis.
References
(1) F.W. Karasek, Anal. Chem. 46, 710A–720A (1974).
(2) D.I. Carroll, I. Dzidic, R.N. Stillwell, and E.C. Horning, Anal. Chem. 47, 1956–1959 (1975).
(3) H.E. Revercomb and E.A. Mason, Anal. Chem. 47, 970–983 (1975).
(4) H.H. Hill, Jr., W.F. Siems, R.H. St. Louis, and D.G. McMinn, Anal. Chem. 62, 1201A–1209A (1980).
(5) G.F. Verbeck, B.T. Ruotolo, K.J. Gillig, and D.H. Russell, J. Amer. Soc. Mass Spectrom. 15(9), 1320–1324 (2004).
(6) R.W. Purves and R. Guevremont, Anal. Chem. 71, 2346–2357 (1999).
(7) R. Guevremont, J. Chrom. A 1058, 3–19 (2004).
(8) M.E. Ridgeway, "Advances in High Field Asymmetric Ion Mobility Spectrometry (FAIMS) Analyzers and FAIMS-Mass Spectrometry Interfaces," PhD dissertation, University of North Carolina, 2010.
(9) B.C. Bohrer, S.I. Merenbloom, S.L. Koeniger, A.E. Hilderbrand, and D.E. Clemmer, Annual Rev. Anal. Chem. 1, 293–327 (2008).
Kenneth L. Busch knew, from his first days working with a GC–MS instrument, that ions were more than dimensionless points of mass and charge. Mass that moved as gas-phase molecules through the chromatography column became mass that moved as ions through the mass spectrometer, ending with a splat at the electron multiplier. Late at night, he could hear the splat. Maybe, in retrospect, this explains a lot. From the perspective of a nanoLilliputian, different sizes and shapes of ions discernible in IMS and FAIMS are a grand display of diversity that points the way to the future. This column is the sole responsibility of the author, who can be reached at wyvernassoc@yahoo.com.

Kenneth L. Busch

Synthesizing Synthetic Oligonucleotides: An Interview with the CEO of Oligo Factory
February 6th 2024LCGC and Spectroscopy Editor Patrick Lavery spoke with Oligo Factory CEO Chris Boggess about the company’s recently attained compliance with Good Manufacturing Practice (GMP) International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) Expert Working Group (Q7) guidance and its distinction from Research Use Only (RUO) and International Organization for Standardization (ISO) 13485 designations.