From Laboratory Technique to Process Gas Sensor: The Maturation of Tunable Diode Laser Absorption Spectroscopy
Tunable diode laser absorption spectroscopy (TDLAS) has evolved from a laboratory specialty technique to the foundation for highly sensitive and specific gas-phase sensors for the process environment. This article discusses two recently developed products, a portable trace gas monitor, and a water vapor flow sensor.

Editor's Note:
Now, I admit that the mention of near infrared spectroscopy makes me start to salivate; nonetheless, this device would have been notable at any wavelength region. As any of you who follow this space know, I am a huge proponent of smaller, faster, and portable instruments for process work. This month, we are treated to a fine article by my friend, Mark Druy (with Mickey B. Frish and William J. Kessler, Physical Sciences Inc., Andover, Massachusetts), who describes an itsy-bitsy gas monitor, based upon a tunable laser, of all things. I read, learned, and enjoyed; I know you will, too.
Introduction
The advent of lower cost near- and mid-infrared spectrometers has facilitated many new gas analysis applications in laboratory and process environments. Several factors govern the success of an instrument's use in a process environment: reliability, reproducibility, robustness, and embedded software that eliminate the need for a trained spectroscopist to analyze the results. In other words, the spectrometer becomes a sensor for a specific parameter that needs to be measured rather than a general purpose laboratory tool.
This article examines how tunable diode laser absorption spectroscopy (TDLAS) has evolved from a laboratory specialty technique to the foundation for highly sensitive and specific gas phase sensors for the process environment. Until the 1990s, TDLAS for gas analysis was suitable for laboratory use only, requiring highly trained individuals to operate high-maintenance devices and provide interpretation of the data. During the past decade, the technology has emerged from the laboratory to become a reliable, robust, and (for selected industrial applications) commercially available means for continuously measuring and, in some cases, controlling extremely small concentrations of selected gas analytes. For the most part, these practical and robust commercial TDLAS instruments came into existence during the 1990s, owing to the advent of reliable single-mode near-infrared (NIR, 1.2–2.5 μm, or 8500–4000 cm-1 ) diode lasers that operate continuously and unattended near room temperature (as opposed to early NIR diode lasers that required cooling by liquid nitrogen). The technology has now been refined to the point where complete TDLAS analyzers are available in lightweight, battery-operated packages similar to a smoke detector and cost only a few thousand dollars.
Background
TDLAS gas analyzers rely on well-known spectroscopic principles. Gas molecules absorb energy in narrow bands surrounding specific wavelengths in the electromagnetic spectrum. At wavelengths slightly different than these "absorption lines," there is essentially no absorption. Specifically, when the laser wavelength (or its reciprocal, the frequency, usually expressed in wavenumbers or cm-1 ) is tuned to correspond to a particular absorption feature of the target gas molecule, the light transmitted across a measurement path containing the target gas is attenuated according to the Beer-Lambert relation:
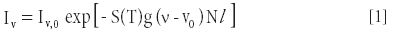
where Iv is the transmitted intensity, ν is the laser frequency, l is the optical path length through the gas, Iv,0 is the initial laser intensity, S(T) is the temperature-dependent absorption line-strength (a fundamental spectroscopic property of the molecule), N is the target species number density, and g(ν - ν0), called the "lineshape" function, describes the frequency dependence of the absorption feature strength.
The argument of the exponential function is the fractional change in the laser intensity across the measurement path and is conventionally known as the absorbance. By transmitting a beam of light through a gas mixture sample containing a quantity of the target gas, tuning the beam's wavelength to one of the target gas's absorption lines, and accurately measuring the absorption of that beam, the analyst can deduce the concentration of target gas molecules integrated over the beam's pathlength. This is schematically shown in Figure 1, where λ0 is the wavelength of the absorption line of the gas and δ is the tuning range of the laser about the absorption line of the target gas.
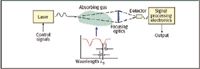
Figure 1: Principles of gas phase spectroscopy with tunable diode lasers.
Typically, each TDLAS system is built using a laser having a specific design wavelength chosen to optimize the sensitivity to a particular target gas. The wavelength is selected to correspond to a specific absorption line of the target analyte gas that is free of interfering absorption from other molecules. In most TDLAS instruments, the laser's fast tuning capability is exploited to scan the wavelength across the selected gas absorption line rapidly and repeatedly. While this scanning occurs, the fraction of laser power that is transmitted through the gas mixture is monitored with a photodetector. When the wavelength is tuned to be off of the absorption line, the transmitted power is higher than when it is on the line. Measurement of the relative amplitudes of off-line to on-line transmission yields a precise and highly sensitive measure of the concentration of the target gas along the path transited by the laser beam.
Furthermore, multipass optical configurations (such as Herriot and cavity-enhanced absorption cells that provide long absorption paths in small physical lengths) frequently are combined with well established noise reduction techniques (known as frequency or wavelength modulations spectroscopy [1] and balanced ratiometric detection [2]) in TDLAS instruments to make them exquisitely sensitive to ultratrace concentrations of target gas. Thus, TDLAS instruments offer a combination of high sensitivity to trace concentrations of many gases, freedom from cross-species and external interference, and fast response. Examples of gases that can be sensed with TDLAS, and typical minimum detectable path-integrated concentrations, are listed in Table I.
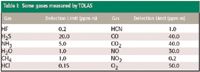
Table I: Some gases measured by TDLAS
Application Examples
Two application examples for TDLAS gas phase sensors demonstrated recently were a portable trace gas monitor and a water vapor flow sensor.
Portable trace gas monitor: Figure 2 shows a complete battery-operated TDLAS system in a package comparable in size and weight to a home smoke detector. The package contains a 2-in. optical measurement path (seen as the silver section in Figure 2) across which the laser beam is projected. The device utilizes the audio output to act as an alarm and provides a DB-9 connector for connection to the serial port. A jack is provided for connecting a battery charger and there is a power switch. The device is activated by merely switching it on and requires less than 10 s to "warm-up" and function. The package can be configured (with minor modification) with an optical fiber connector to allow use of an external measurement path. Potential applications of this device include industrial or commercial toxic gas alarms, on-line trace gas process monitoring and control, environmental monitoring, combustible gas alarms, and combustion gas (fire) sensors. The internal view of the system shows the TDLAS Control Unit incorporating all functions needed for wavelength modulation spectroscopy on a single 6-in. square printed circuit board with digital signal processing (DSP) and an embedded microcontroller. All of the laser control, thermal control, signals processing, and data-reporting functions are performed on this board, which draws only 1.5 W of power. The system typically is powered by two AA sized rechargeable Li-ion batteries, which provide more than 8 h of continuous operation between charges. Battery-charge control circuitry is built onto the circuit board. An audio output also is provided that produces an audible tone having a pitch that is related to measured analyte concentration. This enables the device to be utilized as a simple alarm rather than a quantitative analytical tool.

Figure 2: Complete TDLAS system packaged like a smoke detector.
To use the device as a quantitative analytical tool, a serial port, using the RS-232 protocol, is used for communicating output data as well as for system configuration. In the set-up mode, by communicating via a PC or PDA, the user can adjust laser-operating parameters such as temperature, average current, and modulation depth. These parameters, once set, are stored in RAM and the PC can be disconnected during normal operation. Should the PC remain connected, during normal operation the serial port continuously streams data including measured analyte concentration, system status including any faults or warnings, and other information describing operational parameters. Software on the PC can receive and graphically display this information
Water vapor sensor for lyophilization (freeze-drying) processes: Freeze drying (3) is a process by which a dry solid is produced from a solution by first converting most of the water to solid (ice) during a freezing process, removal of most of the water by direct sublimation during the primary drying phase, and finally, removal of bound water that did not sublimate by desorption during the secondary drying phase. In the production of pharmaceutical products, conversion to the solid state normally is done for stabilization. Many pharmaceuticals, such as vaccines and biotechnology products, do not have sufficient chemical and physical stability to withstand the rigors of distribution and storage in aqueous solution, so conversion to a stable solid is essential to maintain product quality and efficacy.
Freeze-drying is an expensive process largely because process times are long and commercial freeze-drying plants are expensive to purchase and maintain. Freeze-drying is also a complex operation, and largely because of this complexity and the lack of adequate process analytical technology, most commercial freeze-drying processes are suboptimal, thereby wasting time, money, and placing product quality at risk. The measurement and control of product temperature and the timing of the change in shelf temperature from primary drying conditions to secondary drying conditions are critical. In some cases, processes designed in the laboratory do not scale to manufacturing because of dryer mass and heat transfer overload. Water sublimation and desorption rates, and thus water vapor mass flux, are related directly to the product temperature during freeze-drying.
Using the principles of TDLAS, it is possible to develop an on-line sensor for monitoring the water removal rate during pharmaceutical freeze-drying (and other drying processes). By transmitting a TDLAS laser beam through a duct between the freeze-dryer chamber and condenser, it is possible to measure the water vapor concentration (or density) and the gas flow velocity. The principles underpinning the density measurements already have been provided in the "Background" section of this article. The velocity of a flowing gas can be determined by measuring the Doppler-shifted absorption spectrum of a molecular constituent of the gas at a lineofsight angle with respect to the gas flow vector. The absorption spectrum will be shifted in wavelength or frequency with respect to the absorption wavelength of a static gas sample by an amount related to the velocity of the gas and the angle of the probe laser beam with respect to the flow vector. The density and velocity measurements are combined with the knowledge of the duct cross-sectional area to determine the water vapor mass flow rate, or mass flux.
The velocity measurement concept is shown in Figure 3. The velocity from the Doppler-shifted absorption spectrum of the water vapor is derived from the laser propagation vector k at a known angle θ to the velocity vector u. The absorption spectrum will be shifted in wavelength or frequency with respect to the absorption wavelength of a static gas sample by an amount related to the velocity vector of the gas u and the angle between u and the probe laser beam propagation vector k.

Figure 3: Schematic diagram showing the Doppler-shifted absorption spectroscopy velocity measurement concept.
Equation 2 shows the relationship used to determine the gas flow velocity u. c is the speed of light, δω is the peak absorption shift from its zero velocity frequency, ωo is the absorption peak frequency at zero flow velocity, and θ is the angle formed between the laser propagation across the flow and the gas flow vector.

The mass flux is calculated by the product of the measured number density (N g/cm-3 ), the gas flow velocity (u, cm/s), and the cross-sectional area of the flow duct (A, cm2 ). This is shown in Equation (3).
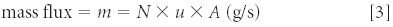
A measurement schematic and a photograph of the optical setup mounted within a laboratory scale freeze-dryer are shown in Figure 4.

Figure 4: Schematic of a mass-flux measurement in a pharmaceutical freeze dryer.
Figure 5 shows the rate of water removal during a sample pharmaceutical product freeze-drying process. The total mass of water removed, as deduced from the TDLAS data, compares remarkably well with gravimetric measurements of the sample gathered before and after the freeze-drying process.
Summary
TDLAS has evolved from a laboratory technique to a robust industrial technology suitable for portable trace gas analysis or in-process measurement of process gases. These new TDLAS instruments are based upon reliable and robust diode lasers that emerged from developments for the telecommunications industry and operate on familiar principles of infrared absorption. By nature of the narrow spectral line widths of the diode lasers and the sophisticated noise-reduction techniques that are used in the detector circuits, TDLAS instruments are highly selective and sensitive in their ability to detect many gases without cross–species or external interference.

Figure 5: Temporal profile of water vapor removed during freeze-drying as measured by the TDLAS sensor.
References
(1) D.S. Bomse, A.C. Stanton, and J.A. Silver, Appl. Opt.31 (1992).
(2) M.G. Allen, K.L. Carleton, S.J. Davis, W.J. Kessler, C.E. Otis, D. Palombo, and D.M. Sonnenfroh, Appl. Opt.34, 3240 (1995).
(3) M. Pikal, "Lyophilization," Encyclopedia of Pharmaceutical Technology, vol. 6, J. Swarbrick and J.C. Boylan, eds. (Marcel Dekker, New York, 2001).
Acknowledgments
The authors wish to acknowledge the support of their colleagues at Physical Sciences, Inc. to the development of the products mentioned in this article.
They also wish to acknowledge the contributions of Professor M. Pikal and Dr. H. Gieseler, University of Connecticut, Storrs, Connecticut, to the development of the freeze drying water vapor sensor.

New Near-Infrared Machine Learning Technique Identifies Dangerous Blood for Transfusion Safety
May 6th 2024Researchers in China have developed a cutting-edge machine learning approach that can detect chylous blood in blood intended for transfusion with more than 90% accuracy. This development promises to significantly reduce the risks associated with blood transfusions and improve the efficiency of blood donation centers.
New Probes for NIR Monitoring of Polymer Injection Molding Composition in Real-Time
May 2nd 2024Researchers from Kyoto University and Japan's National Institute of Advanced Industrial Science and Technology have developed innovative probes to monitor the chemical composition of biodegradable polymer blends during injection molding. This breakthrough could lead to improved production efficiency and reduced waste in the polymer industry.