Process Monitoring: In-line, At-line, or Slip-stream?
As process analytical technology (PAT) moves out of the laboratory and into the plant and to the process stream itself, the question arises, "What is the best way to collect data from stream samples?" The author shows that this depends upon both the nature of the stream and the components to be measured.

Process sensors fall into three general categories:
- Environmental sensors — measure environmental conditions in the stream (such as pressure, temperature, and flow rate)
- Physical property sensors — measure pH, conductivity, density, refractive index, and viscosity
- Stream composition sensors — determine the presence and identity of components in the stream and their concentrations
Environmental and physical property sensors provide information about the stream as a whole — not about the individual components in the stream. A stream can have only one refractive index or conductivity at a time, which can vary as stream composition varies, but neither the environmental sensors nor the physical property sensors indicate which component or components are causing the change.

Figure 1. An in-line IR carbonation sensor on a beer production line. For this particular application only an in-line sensor can monitor dissolved CO2 in situ because the carbonation escapes as soon as the pressure is released.
To supply the same sort of information for stream, reaction vessel, or fractionation tower that is provided by analyzing samples brought to analytical instruments in the laboratory, compositional sensors are required. In the laboratory, gas chromatographs, gas chromatography (GC) mass spectrometers, and optical spectrometers — UV, visible, and infrared (IR) — are the prime composition information-gathering instruments used. However, none of these instruments is comfortable in the environmental conditions of the process plant, let alone capable of making measurements in-line or at-line.
To meet the environmental and continuous measurement requirements of process analytical technology (PAT), a whole new class of sensing equipment is evolving. For the most part, they are based upon spectroscopy techniques — UV and IR, both near and mid. Following are several reasons for the choice of spectroscopy over GC or mass spectrometry (MS):
- Unlike GC or MS, optical spectrographic measurements are nondestructive so that sensors can be placed in or near sample streams without upsetting the streams.
- All organic and most inorganic molecules have characteristic mid-IR spectra that can serve to identify them and, because IR spectra are additive, measuring the strength of key absorption bands can quantify the various components in a mixture.
- The availability of optical filters (both narrow band pass and linear variable) and rugged multielement detectors that do not require cooling, are enabling the construction of simple, rugged devices that meet the environmental requirements of the processing plant and are capable of providing the desired component concentration data.
A New Sensor
Now the question is, "How do we make use of these new sensors to gather stream data?" As mentioned earlier, there are three different approaches to gathering IR absorption data — in-line, at-line, and slip-stream. At first glance, it would appear that the in-line approach would be the most logical and desirable. However, process streams come with a wide variety of conditions, all of which must be evaluated to determine whether it is feasible to put a sensing probe in-stream, or whether better results can be achieved with one of the other sampling procedures.
A typical in-line IR sensor consists of an optical element, transparent in mid-IR, with a source and detector positioned in such a way that energy is reflected internally from the source to the detector. The optical element can assume the shape of a prism, trapezoid, truncated cone, or hemisphere. When placed in a process stream, energy will be absorbed from the optical element by means of attenuated total reflection (ATR) at wavelengths where the liquids in contact with the optical element absorb.

Figure 2. "At-line" process stream monitoring. The black spectrum above is that of a sample taken from the process stream. The red spectrum is that of the residual materials left after the evaporation of 100 μL of stream sample. From this data, the concentrations of the three raw materials and that of the material being synthesized in the process, can be determined precisely in approximately 3 min.
Under ideal stream conditions, these IR probes can provide continuous and accurate data on the components that make up the stream. However, stream conditions are not always ideal — the temperature might be too high (above 60 °C), material might plate out on the sensor surface, or cleaning materials might affect the optical element. The net result is that these sensors require either a routine method for resetting the zero, or some other approach to process monitoring.
Two very successful applications of in-line IR sensors are the monitoring of carbonation levels in beer and soft drinks and Brix (sugar) levels in soft drinks. In cases in which in-line sensing is impractical, the availability of small, rugged IR spectrometers makes it possible to place them close to the process stream. For liquid measurements by mid-IR, ATR sample stages usually are used. A few drops from the process stream placed on the ATR sample stage result in analytical data being available in a minute or so.
If, as is often the case, the solvent in the stream is strongly absorbent, making it difficult to see the spectra of the other components through its spectrum, it is possible to deposit ~100 μL of stream sample on the ATR surface, letting the solvent evaporate and taking the spectra of the residual components. When known quantities of sample are deposited, accurate measures of the individual stream components can be determined after the solvent is evaporated, usually in about three minutes.
There might be certain conditions under which taking a slip-stream off the main stream will produce the best results. For example, too high stream temperatures can be corrected by cooling the slip-stream. Significant amounts of particulate matter can be filtered out if they interfere with spectral measurement.
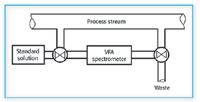
Figure 3. Slip-stream sample system diagram. A typical slip-stream sample system permits either an off shoot from the main process stream or standard solutions for calibration purposes to be passed through a portable IR spectrometer. Changes can be made in the spectrometer calibration through the use of standard solutions when changes are made in the main stream calibration. Samples either can be returned to the main line of sent to waste.
In the case of beverage monitoring, it was found that sugar tended to plate out on the in-stream ATR element at the customarily low temperature (4 °C) of a beverage line. With a slip-stream, the temperature could be brought up to about 20 °C to eliminate the plating.
Slip-streams serve another purpose: With a variable filter array (VFA) spectrometer mounted along side the process stream, incorporating a slip-stream sample stage, changes in basic stream composition can be corrected for by passing a set of calibration standards through the VFA sample stage off line and then returning it to the slip-stream ready to accept the new formula.
Slip-streams generally are returned to the main stream after analysis. However, if there are sanitary requirements, they can be sent to drain with minimum loss because generally, they have a very small volume.
Conclusion
As has been shown, IR sensors are versatile and generally can be adapted to almost any stream environment. A few examples are described in Figures 1–3.
Even though in-line sensors appear to be the most attractive, the environment in which they are to be used should be considered carefully to make sure it is amenable to in-line measurements. As pointed out earlier, there might be conditions under which other approaches provide more usable information.
Paul Wilks is president of Wilks Enterprise, Inc. (South Norwalk, CT).
