X-Ray Technology Today: An Overview
The twentieth century saw the rise of several novel instrumental techniques based on the use of X-rays. Today, X-ray spectroscopy and diffractometry continue to prove their utility as advances in instrumentation produce new methods and enable new applications.

Tince the discovery of X-rays in the late 19th century, this band of the electromagnetic spectrum has been a fertile source of scientific innovation. Less than a year after William Conrad Roentgen's discovery, X-rays were being used in medicine for the purposes of radiography. Due primarily to the medical uses of X-rays, Roentgen was awarded the first Nobel Prize in Physics in 1901, but beyond the medical and imaging uses of X-rays, the Nobel presentation speech presciently noted that "there is no doubt that much success will be gained in physical science when this strange energy form is sufficiently investigated and its wide field thoroughly explored." In the ensuing century, X-rays were adapted for use in spectroscopy and chemical analysis in several ways that revolutionized entire fields of scientific inquiry.
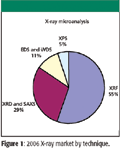
Figure 1
By far the most common X-ray–related technique is X-ray fluorescence (XRF) spectroscopy, in which X-rays eject electrons from the inner electron shells of atoms in the sample. As the electrons make a quantum transition from an outer electron shell into the vacated state, a photon is emitted. This secondary radiation is analyzed by a spectrometer in order to identify the relative abundance of the elements. The initial development of this technique was carried out by Manne Siegbahn, who was awarded the Nobel Prize in Physics in 1924.
There are two main spectrometer types used in XRF, energy dispersive (ED-XRF) and wavelength dispersive (WD-XRF). Roughly speaking, ED-XRF is less expensive and requires a much less powerful X-ray source, but WD-XRF has greater resolving power and precision while also being less prone to interferences. Depending on the particular application, either technique may be preferred.
Perhaps the greatest change in XRF over the past decade has been the explosive growth in handheld XRF instruments. Because of the size and power limitations, these instruments are all ED-XRF instruments. Originally, many handheld instruments actually used radioactive sources to produce the X-rays. Now, however, the development of small X-ray tubes has eliminated the use of radioactive substances as well as the risks and regulations surrounding them. Handheld instruments have been used in applications like scrap-metal identification or positive material identification. But more recently, handheld XRF has become very popular among manufacturers that have decided that handheld XRF is an excellent screening tool for hazardous materials regulations that have recently gone into effect, such as the European Union's Restriction of Hazardous Substances (RoHS), which went into effect in July 2006. The RoHS directive calls for strict limits on the amount of cadmium, lead, and other hazardous substances in electrical and electronic equipment. Although laboratory XRF still provides the benchmark standard for these analyses, handheld systems have aided manufacturers in their compliance activities. Companies involved in the handheld XRF market include Thermo Fisher Scientific (Niton) (Waltham, Massachusetts), Innov-X (Woburn, Massachusetts), and Bruker AXS (Billerica, Massachusetts), which recently acquired Keymaster Technologies.
Another relatively novel form of commercial XRF is the X-ray microscope. Instruments such as the Horiba XGT-5000 (Horiba Jobin Yvon, Edison, New Jersey) combine an optical microscope with an XRF analyzer so that researchers can use the optical imaging to guide their elemental analysis. The X-ray optics provide a small spot size, with spatial resolution of about 10 μm.
Some improvements and modifications of the basic XRF technique have been introduced to counteract some of the difficulties of the technique, mainly the background noise. One such method is to alter the geometry of the instrument so that the X-rays impinge upon the surface at a grazing angle. Most of the high-intensity beam is directed away from the detector, producing measurements with less background. Although total reflectance XRF, as it is known, has advantages, it also requires a very carefully prepared flat surface, making it unsuitable for many samples. A less restrictive method is to use a polarized beam of X-rays as the excitation source. There are commercial instruments on the market that offer these possibilities. Other advances in XRF spectroscopy involve improvements in the sources, detectors, and software. Probably the most significant change has been in the detectors. Although silicon lithium Si(Li) detectors historically have had better performance, particularly for the lighter elements where XRF has difficulties, the improvements in silicon drift detectors have narrowed that performance gap while offering advantages such as higher count rates. Silicon drift detectors can be cooled electronically, while Si(Li) detectors typically require liquid nitrogen cooling.
Closely related to XRF are surface analysis systems that use X-rays as a probe. For instance, energy dispersive or wavelength dispersive spectrometers are a common attachment to electron microscopes. Unlike XRF, for these analyses the X-ray signal is created by the electron beam impinging on the surface. The analysis is quite similar, and advances in detector design similar to those for laboratory XRF have helped improve these instruments as well. Almost the reverse of this process is found in X-ray photoelectron spectroscopy (XPS), also known as electron spectroscopy for chemical analysis (ESCA). In this technique, X-rays probe the surface, causing the release of secondary electrons. By measuring the energies of the emitted electrons, the composition of the surface can be determined.
Quite apart from standard spectroscopy, X-rays also ushered in an entirely new field of physical inquiry with the discovery of X-ray diffraction (XRD). X-rays, which have a wavelength comparable to the interatomic spacings of the sample, diffract off the atoms of the sample. In a crystal, the regular spacing of the crystal lattice gives rise to well-defined diffraction patterns that encode the physical structure of the crystal. Two more Nobel Prizes were awarded for the discovery and explanation of these XRD patterns by von Laue (1914) and the Braggs, father and son (1915).
Applications for XRD range widely from mechanical engineering to pharmaceutical research. Residual stress testing makes use of XRD's sensitivity to lattice spacings to locate discontinuities or other signs of stress in metal components. XRD is an interesting method for such testing, since it is nondestructive and components can often be examined in situ. Residual stress testing is typically used in engineering or military applications. More ordinary uses of XRD focus on crystal structures in materials or, increasingly, on pharmaceutical molecules and proteins. In single-crystal XRD, the sample is a relatively large crystal of the substance under examination; usually these are large biological molecules, such as proteins or viruses, and suitable crystals are often difficult to prepare. In powder diffraction, a powder of small crystals is used. Although the crystals in the powder have random orientations, a useful diffraction pattern can still be achieved. Both kinds of analysis provide information on the structure of the molecules in question. For the pharmaceutical industry, XRD is helpful in testing the stability of drug formulations by distinguishing phase transitions between different crystal phases. XRD is also in great demand in academia, and the requirements of studies of the biomolecular structures are pushing researchers toward more elaborate systems with better sensitivity and faster analyses.
Most of the advances in XRD involve X-ray sources and X-ray detectors. XRD generally requires a higher X-ray flux than XRF instrumentation. Indeed, some high-resolution studies of minuscule samples or short-lived chemical interactions benefit from using synchrotron radiation as the X-ray source. However, few researchers are fortunate enough to have access to a synchrotron. On the more commercial level, X-ray tubes are a more common source, with rotating anode tubes being more common for XRD because of their higher flux. Through improvements in anode design and X-ray optics, modern XRD sources generate both higher powers and tighter spots, resulting in greatly increased flux. Some of the more recent sources on the market even rival the capabilities of older synchrotron beamlines.
For detectors, there are many different parameters that are important for high-resolution analysis: angular resolution, maximum count rate, and dynamic range. There have been improvements in all of these factors over the last few years. But probably the greatest advance has been in increasing the dimension of the detector. By moving a point detector, one can build up a diffractogram, although rastering over a wide range of solid angle naturally takes a significant amount of time. Line detectors have become quite common in XRD, and this reduces the analysis time significantly. The development of two-dimensional detectors with comparable performance has now been achieved, and a complete diffractogram can be achieved without moving the detector at all. This has enabled high-throughput XRD for many routine applications.
Small angle X-ray scattering (SAXS) is closely related to XRD and has been an area of growth over the past decade as dedicated and dual systems have become available. In SAXS, X-rays are scattered off the sample at angles of just a few degrees. The scattered X-rays contain information on the structure of macromolecules on the nanoscale. Unlike XRD, SAXS can be used with noncrystalline materials, which makes it useful in a broader range of applications.
Combined, the market for X-ray instrumentation was about $1 billion in 2006, and XRF and XRD continue to grow rapidly. The future will no doubt bring further advances and elaborations of all these techniques. One potential innovation that could revolutionize crystallography and other techniques would be a reliable and affordable X-ray laser. Although sources of coherent X-rays have been demonstrated, it may be many years before they become commonplace equipment in the laboratory.
Mike Tice is Vice President of Consulting Services at Strategic Directions, International (Los Angeles, California).

University of Pennsylvania Graduate Researcher Wins SPIE Medical Imaging Student Paper Award
March 14th 2024A PhD student in the Department of Bioengineering at the University of Pennsylvania has won the 2024 Physics of Medical Imaging Student Paper Award, which is given out annually by the International Society for Optics and Photonics (SPIE), at the Medical Imaging Symposium in San Diego, California.
Advancements in Non-Invasive Analysis of Historical Metal Artifacts
December 19th 2023Studying historical ancient artifacts requires the use of a nondestructive technique to analyze the metal surfaces of these objects. This study presents two approaches that improves on existing methods when conducting alloy analysis.
Unraveling Polyester Fibers with Advanced X-Ray Techniques
December 5th 2023Researchers at Kochi University and RIKEN have unveiled a new method for distinguishing individual polyester fibers in forensic investigations. Published in Spectrochimica Acta Part B: Atomic Spectroscopy, their advanced X-ray analysis refreshes how we unravel the composition of these fibers.