Article
Spectroscopy
Spectroscopy
Fluorescent Lights
A look at how fluorescent lights are spectroscopically different from the old-fashioned incandescent light bulb.

In the past, I've done columns on source lamps and light-emitting diodes (LEDs), but there's one kind of light that is becoming more popular in our everyday lives — the fluorescent light. Here, we'll look at how these lights are different, spectroscopically, than the old-fashioned incandescent light bulb.
Thomas Edison wasn't the first person to work on incandescent light bulbs — indeed, as early scientists as Humphrey Davy and Alessandro Volta tried to use electricity to heat a substance to glowing hot. However, Edison was the first to make a practical, commercially viable light bulb. As culture-changing an impact as incandescent light bulbs were, they have one major problem: inefficiency. As much as 90% of the energy given off by an incandescent light bulb is heat. That may be useful if you live at the North Pole, but in most temperate climes it simply adds to the temperature increases that must be combated with air conditioning. Incandescent light bulbs are not optimal sources of light.
There is more than one way to generate light, though. It uses ideas of quantum mechanics instead of thermal physics.
Fluorescence
Fluorescence is a process by which a substance absorbs light and then emits light of a different wavelength. In most cases, fluorescing materials emit light of lower frequency and energy than is absorbed, although there are occasionally two-photon emissions in which the emitted light is a higher energy. The word "fluorescence" was coined by British physicist G.G. Stokes in 1852 after the mineral fluorite (crystalline CaF2), which fluoresced strongly because of impurities. It was observed as early as the 1560s, but it was only in the mid-19th century that Stokes described the phenomenon after experimenting with ultraviolet light (which itself was only identified as part of the spectrum in 1801).
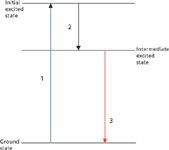
Figure 1: Schematic of the fluorescence process: 1 = excitation, 2 = relaxation, and 3 = emission. The initial and intermediate excited states can be different electronic states or even two different states within the vibrational manifold of the same electronic state. See text for details.
The mechanism of fluorescence had to wait until the understanding of quantized energies in atoms in molecules, but a simplified version of the mechanism is shown in Figure 1. An atom or molecule absorbs a photon of light (step 1 in Figure 1). In the finite but brief time the system is in the excited state, it loses energy via some mechanism, like collisions with solvent molecules or vibrational energy transfer to neighboring atoms or molecules. This step (step 2 in the figure) is generally referred to as "nonradiative relaxation" or "radiationless decay." The energy loss stops at some intermediate but lower energy state. Then, the system emits a photon and returns to the ground (or some other lower) state (step 3 in the figure). Because the intermediate state is lower in energy than the initial excited state, the emitted photon is lower in energy than the exciting photon, leading to an apparent wavelength or color shift; this is called the Stokes shift in honor of the aforementioned British physicist. Finally, in fluorescence processes, the energy states involved have the same multiplicity (that is, overall electron spin), so the shifts between states are quantum-mechanically allowed and so are fairly fast — on the order of nanoseconds. Thus, we experience fluorescence processes as immediately connected to the presence of source of the exciting photons. (Contrast that to phosphorescence, which involves a spin-forbidden transition and therefore is relatively slow, having lifetimes on the order of minutes or hours.)
Many minerals and organic molecules fluoresce. Geology uses fluorescence to help identify certain minerals and gemstones. Quinine, a natural antimalarial compound found in the cinchona tree, fluoresces, as does petroleum jelly. Green fluorescent protein (GFP) is a 238-amino acid protein used widely in molecular and cell biology; its developers won the 2008 Nobel Prize in Chemistry as a tribute to its importance. Fluorescence spectroscopy is a major type of spectroscopy in its own right — but that's another column.
Fluorescent Lights: Development
In 1856, German glassblower Heinrich Geissler invented a mercury-based vacuum pump that could evacuate glass better than previously achieved. When an electric current was passed through the tube, the residual mercury vapor in the tube glowed a bright green. (The vapor pressure of Hg at room temperature is about 0.002 Torr, so this was the best vacuum Geissler could get at the time.) The presence of other trace gases in these so-called Geissler tubes could produce other colors, so they became popular amusements. Later, production of better vacuums decreased the amount of light produced, but the Geissler tubes were the precursors to cathode ray tubes (CRTs) which were the basis of the tube television; Crookes tubes, in which experiments with led to the discovery of the electron; and fluorescent lights.
In 1859, Edmond Becquerel (father of Henri Becquerel, who discovered radioactivity) coated a Geissler tube with a fluorescent material, making the first rudimentary fluorescent light. However, it did not work for long and gave off only very feeble light. Although Edison and Nikolai Tesla tinkered with similar systems, it wasn't until 1895 that Daniel Moore, a former employee of Edison's, constructed a workable fluorescent light using carbon dioxide as the emitting substance. It was about three times as efficient as the incandescent light bulbs of the time, and ironically spurred the development of more efficient incandescents, which eventually forced the Moore lamp out of the market.
In 1901, American engineer Peter Cooper Hewitt patented a discharge tube based on mercury vapor, similar to the original Geissler tube. However, the light it gave off was heavy in the blue and green, yielding an unnatural light color. On the other hand, they were much more energy-efficient, using much lower voltages to produce the same brightness as an incandescent light. Development of mercury vapor–containing tubes continued, but mostly in Europe. By the 1930s, coatings of fluorescence materials were used to correct the color and increase the amount of visible light emitted, as well as a ballast to regulate the current during the initial stages of operation. Commercial sale of acceptable, relatively modern fluorescent lights began in 1938 by General Electric, and by the 1950s more light was generated in the United States by fluorescent lights than by incandescent lights.
Modern Fluorescent Lights
Modern fluorescent lights (Figure 2) are anywhere from a few inches to several meters long. Typically, a fluorescent light has a few milligrams of mercury in it that must be vaporized for the light to work properly. The light is also filled with a few torr of an inert gas like neon or argon — not too much, or else the gas inside the bulb will be so resistive that an electrical current won't be able to pass. The inside of the bulb is coated with a phosphor (a rather strange term for a material in fluorescent lights, but the word "fluoror" sounds funny) that is usually a doped metal salt. Older phosphors for fluorescent lights are (Sr,Mg)3 (PO4)2 doped with tin and Ca5 (F,Cl)(PO4)3 doped with antimony and manganese; a variety of rare-earth metal salts, like terbium- and cerium-doped LaPO4 combined with Y2O3 doped with europium are used in modern fluorescent lights.

Figure 2: Several models of modern fluorescent lamps (Getty Images).
When it is turned on, the electrodes in a fluorescent light generate electrons, which collide with mercury atoms and excite the electrons in the mercury. These electrons fall back down to their ground state, releasing light. As the light generates ions, its conductivity increases, so the current must be regulated by a ballast to limit the current. But as mentioned above, much of the light generated is in the ultraviolet and blue end of the spectrum. This light excites the phosphor coating on the glass bulb, which fluoresces at an efficiency of greater than 80% — that is, 80% of the UV photons are converted into visible light photons (the rest are converted to heat). The combined spectrum of the mercury plus the phosphor yields the characteristic light of a fluorescent bulb. Fluorescent lights convert more than 20% of the electrical energy to light, which is 10 times more efficient than incandescent light bulbs. Furthermore, they generate only about one-third of the heat that an incandescent light bulb generates, significantly reducing the heat production for the same amount of light production.
Although fluorescent light approximates natural, white light, the spectrum of a fluorescent light is not the continuous spectrum of an incandescent light. Figure 3 shows a comparison of the two types of bulbs. The incandescent light gives off a continuous spectrum as it approximates a blackbody. The fluorescent light, however, is composed of broad but discrete parts of the spectrum. This is what accounts for the perceived difference between the output of the two different types of light bulbs.
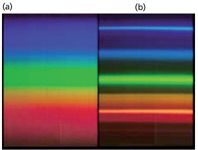
Figure 3: Comparison of the spectra of (a) an incandescent light and (b) a typical fluorescent light. The incandescent light gives a continuous spectrum, while the fluorescent light gives discrete lines typical of the mercury spectrum and the phosphor.
(Want a quick way to tell if a light is incandescent or fluorescent? Use a CD or DVD to generate a spectrum of the bulb — the tiny grooves in the disk act like a grating. If the light is incandescent, you'll see a full spectrum. If the light is fluorescent, the spectrum will be separated into specific colors, much like in Figure 3. Try it! It won't hurt the disk.)
Compact fluorescent lights (CFLs) have become all the rage to replace regular light bulbs in lamps. Although first constructed in the mid-1970s, they weren't commercially available until the mid-1990s and have been increasingly popular ever since. Why did it take so long for them to become commercially viable? Because new ballasts had to be developed for such small bulbs. The standard 4-ft fluorescent bulb needed, well, 4 ft to be installed, and the ballast could be similarly large. But to put a fluorescent light bulb into a table lamp required the ballast to be much smaller if the entire construction was to replace your standard 100-W incandescent bulb.
David W. Ball is a professor of chemistry at Cleveland State University in Ohio. Many of his "Baseline" columns have been reprinted as The Basics of Spectroscopy, available through SPIE Press. Professor Ball sees spectroscopy from a physical chemistry perspective, because that's his background. He recently served as Distinguished Visiting Professor at the US Air Force Academy, but is now back home in Ohio. He can be reached at d.ball@csuohio.edu.

David W. Ball
Newsletter
Get essential updates on the latest spectroscopy technologies, regulatory standards, and best practices—subscribe today to Spectroscopy.





