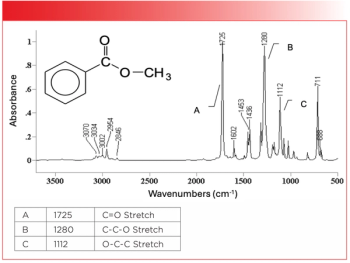
- Special Issues-08-01-2008
- Volume 0
- Issue 0
Rapid Identification of Illicit and Prescription Drugs Using FT-NIR Spectroscopy
The authors discuss the use of FT-NIR to identify several different types of drug formulations.
Near-infrared (NIR) spectroscopy has unique capabilities as compared with mid-infrared analysis because NIR does not require extensive sample preparation to obtain a spectrum. A Fourier transform (FT)-NIR instrument, a novel sample accessory, and a modified search routine for the spectral collection and identification of several different types of drug formulations are outlined, including the examination of illegal drugs.
Infrared and Raman analysis are valuable techniques for sample identification because they provide spectra specific to sample compounds. However, there are limitations to these techniques for the analysis of multicomponent samples because the multiple components often can obscure spectral peaks specific to the compound of interest. For the identification of pharmaceutical preparations, mid-infrared analysis can require extensive sample preparation to obtain spectra that can be searched against a spectral library. For either method, the presence of excipients used for tablet compounding can perturb spectral search routines, leading to incorrect identification of the primary active ingredient, which can also be present in low concentrations. This incorrect or improper identification using search databases can require further interpretation or extensive data processing of the spectral results, namely, subtraction of the excipient components. Illegally manufactured drug compounds are particularly prone to misidentification due to the use of nonstandard excipient compounds (materials for compounding of the tablets or the use of "cutting agents" used to increase the weight of the illegal drug). Near-infrared (NIR) spectroscopy, however, is a nondestructive analysis method that can be used to examine a specific sample area or provide an average of a larger sample area, depending upon the required analysis. NIR also does not generally require sample preparation for spectral collection, and the technique can be relatively insensitive for excipient materials or other contaminants. While the spectra might not be readily interpretable, chemometric analysis of the data can provide quantitative analysis as well as interpretive data with excellent precision. In recent years, NIR spectroscopy has been used widely for the analysis of biological samples and quality control and analysis of food and medical products. In particular, the diffuse reflectance technique is ideal for NIR analysis due to its simple sample handling compared to other analysis techniques. This allows rapid data collection as well as identification of drug components, similar to those methods used for process analytical technology (PAT). The use of the diffuse reflectance method in addition to chemometric processing of the data can provide a simple and rapid method for analysis and identification of various pharmaceutical and illegal drug preparations.
Experimental
A diffuse reflection accessory (JASCO model VIR-NRF-N, Easton, Maryland) was used in combination with a portable Fourier transform (FT)-NIR spectrometer (JASCO model VIR-9650) for all sample analyses.
A drug tablet or other formulation, such as a suspected MDMA tablet, was placed directly on the sample holder and a measurement was performed without further sample preparation. An InGaAs detector was used to provide enhanced sensitivity and rapid scanning capability for obtaining the data within 10 s of placing the drug compound on the accessory sampling area. Principal components analysis (PCA) was performed to simplify positive identification of the various drug tablets.
Figure 1
The various NIR spectra were analyzed using a PCA program. When the grouping of the spectral data was confirmed based upon the PCA program, a library was established using the NIR spectra. For establishing the PCA data library, 40 types of tablets were repeatedly analyzed, including 25 types of over-the-counter (OTC) pharmaceuticals, such as gastrointestinal drugs, one type of amphetamine (AP), eight types of methylenedioxymethamphetamine (MDMA; street name: ecstasy), three types of methamphetamine (MA), and three types of 3,4-methylenedioxyamphetamine (MDA; street name: the love drug). The utility of a simple identification system was examined by the investigation of the PCA searching algorithm, the calculation parameters, and a threshold established by comparison of the search results from randomly selected tablets. Various drug tablets were placed directly on the diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS) sample holder (Figure 1). In the case of an extremely small tablet that was unable to be placed on the holder, the sample was placed in a test tube for analysis and placed into a powders sampling accessory (Figure 2).
Figure 2
Experimental Results
Figure 3 illustrates the PCA results for the four classes of amphetamine street drugs, including MA, AP, MDA, and MDMA. Figure 4 includes examples of the FT-NIR spectra for the four classes of illegal drugs.
Figure 3
Even though the illegal drugs have similar molecular structures, sample identification is easily accomplished by the PCA algorithm as well as the spectral differences. Figure 4 also demonstrates differences in the NIR spectra where the spectral absorptions offer specific peaks depending on the tablet components. The PCA method, however, provides distinct discrimination between the various drug components due to subtle variations in the NIR spectra, even though the different drug formulations contain various excipient materials. Because NIR spectra do not provide specific discriminatory peaks like mid-IR spectra, the PCA method offers an enhanced discrimination of the various drug components, strengthening the identification of the "unknown" drug tablet. The NIR diffuse reflection system as outlined is ideal as a rapid analysis method because the tablet is simply placed on the accessory platform and the required sample measurement time is only 10 s. This method will be enhanced for sample identification as the library data is expanded with additional standard sample data.
Figure 4
Additional spectra were collected from the various MDMA tablets, and the tablets were then analyzed using gas chromatography (GC) to establish the MDMA component concentration. Figure 5 demonstrates a calibration model illustrating the correlation between tablets containing MDMA and the quantitative results of these tablets using GC analysis. With a correlation coefficient of R = 0.966, these results demonstrate a sufficient correlation for performing quantitative analysis of the various MDMA samples. These results indicate that it is possible to analyze the amount of MDMA in illicit tablets by linking the search results of the identification program with the calibration model developed using the GC analysis. Figure 6 is an example of the program developed for the identification and quantitation of the MDMA drug formulation using the PCA search method. The results in the figure also list the amount of the active ingredient in the tablet formulation as calculated from the NIR–GC calibration model. Figure 7 demonstrates the PCA search method as applied to an OTC cold remedy, demonstrating that the NIR DRIFTS method and the PCA search program can also be used to discriminate among various prescription drugs, including the determination of counterfeit formulations.
Figure 5
Figure 6
Conclusions
We have demonstrated the use of an NIR analysis method utilizing PCA discrimination for the identification of various drug formulations. The NIR system demonstrates a rapid, nondestructive analysis method for various drug tablets that can be extended to provide quantitative analysis of the drug concentration.
Figure 7
Richard A. Larsen is with Jasco, Inc., Easton, Maryland. Ken-ichi Akao and Ms. Chihiro Jin are with Jasco Corporation, Tokyo, Japan.
Articles in this issue
over 17 years ago
Integrated FT-IR and Raman Imaging on the Same Microscopeover 17 years ago
Microbial Identification Using FT-IRover 17 years ago
The Imaging Advantage to FT-IR SpectroscopyNewsletter
Get essential updates on the latest spectroscopy technologies, regulatory standards, and best practices—subscribe today to Spectroscopy.