Rapid Multielement Nanoparticle Analysis Using Single-Particle ICP-MS/MS
Spectroscopy
Complex isobaric and polyatomic spectral interferences can be mitigated using triple quadrupole ICP-MS (ICP-MS/MS) with a collision–reaction cell (CRC). This configuration allows for the multielement characterization and detection of smaller nanoparticle sizes.
There are well recognized difficulties in measuring nanoparticles (NPs) accurately by conventional single-quadrupole inductively coupled plasma–mass spectrometry (ICP-MS), because many natural and engineered NPs are based on elements that are challenging to measure at trace level by ICP-MS, such as iron, titanium, and silicon. This month's "Atomic Perspectives" column discusses how complex isobaric and polyatomic spectral interferences can be mitigated using triple-quadrupole ICP-MS (ICP-MS/MS) utilizing a collision-reaction cell (CRC), allowing for the multielement characterization and detection of smaller nanoparticle sizes.
Metal-based engineered nanoparticles (NPs) are widely used in industrial and technological processes, medical applications, foods, and personal care products. NPs exhibit unique physical and chemical properties compared to bulk chemicals, because of the large surface area of particles with one or more external dimensions ranging in size from 1 to 100 nm. While scientists and specialty chemical suppliers are beginning to harness these novel properties to develop new applications, much remains unknown about the impact of NPs on biological and aquatic systems. Given the potential for NP release during production, use, or disposal, reliable measurement methods are required to assess the concentration of NPs in a range of samples.
Single-particle inductively coupled plasma-mass spectrometry (SP–ICP-MS) is fast-becoming an accepted technique for the characterization of NPs in liquid samples (1). The technique provides valuable information on the mean size, size distribution, and number of nanoparticles in the sample, as well as the concentration of the element dissolved in the solution. A number of early studies used conventional single-quadrupole ICP-MS instruments to characterize simple, spherical nanoparticles composed of a single element, such as silver, gold, or zirconium (2,3). However, large quantities of NPs are manufactured from various oxides of iron, zinc, titanium, and silicon. Because these NPs are composed of elements that are much more difficult to measure at trace levels by conventional ICP-MS, and because of overlaps from polyatomic interferences, SP-ICP-MS methods have been developed using triple quadrupole ICP-MS (ICP-MS/MS) (4).
Principles of Triple Quadrupole ICP-MS
In ICP-MS/MS, an additional mass filter (Q1) is located before the instrument's collision–reaction cell (CRC), enabling a double mass selection capability known as MS/MS mode. Q1 controls the ions that can enter the cell, ensuring that the reaction processes in the cell are controlled and consistent. This extra selection step enables the more predictable and reliable removal of interferences on elements such as aluminum (Al), sulfur (S), and iron (Fe), using reactive cell gases, compared to conventional ICP-MS (5).
When using reactive gases, ICP-MS/MS avoids isobaric and polyatomic interferences by utilizing either on-mass or mass-shift mode. In on-mass mode, the target isotope is measured at its original mass, and the interference or interferences are shifted to a different mass. In mass-shift mode, the target isotope is measured as a reaction product ion at a different mass, free from the interference. This is exemplified in Figure 1, which shows how ICP-MS/MS on-mass mode avoids polyatomic interferences from argon ion (ArO+), calcium ion (CaO+) and iron ion (Fe+) at m/z 56 via proton transfer with ammonia in the CRC. Figure 1 also demonstrates how ICP-MS/MS mass-shift mode for titanium (48Ti) avoids an isobaric interference from 48Ca+ because Q1 allows only ions at m/z 48 to pass to the cell, while all other ions are rejected. 48Ti+ is converted to 48Ti16O+ in the cell with O2/H2 reaction gas mixture. Q2 measures TiO+ at m/z 64, while 48Ca+ reacts to form CaOH+ at m/z 65, which does not interfere with TiO+ at m/z 64.
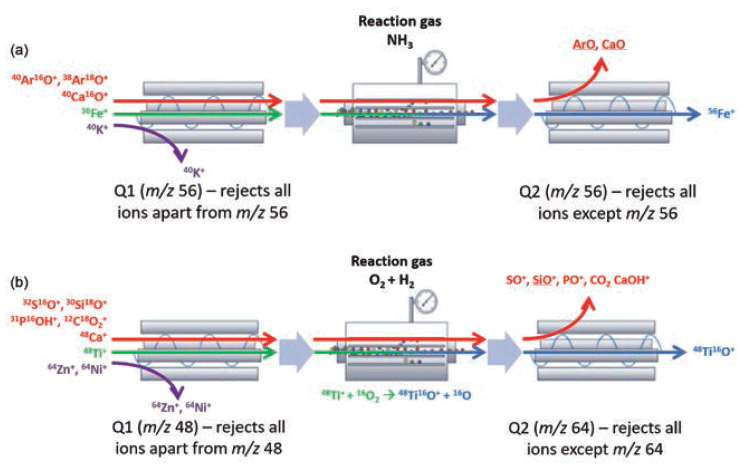
Figure 1: A schematic to show how (a) the on-mass mode, and (b) the mass-shift mode are used in triple quadrupole ICP-MS (ICP-MS/MS) CRC to remove interferences.
Multiple Element Nanoparticle Characterization
To date, most SP-ICP-MS applications have been used to determine samples with a known NP content, but many natural samples contain mixtures of NPs of unknown or variable composition. Also, some NP samples contain more than one metal. In this study, SP-ICP-MS has been further developed to determine multiple element NPs in samples containing a mix of NPs using ICP-MS/MS to provide high sensitivity while removing interferences that can be challenging to eliminate using single-quadrupole ICP-MS. The method has the potential to more fully characterize the NP content of products, chemicals, and environmental samples, as part of a routine monitoring program.
Rapid Data Acquisition
With appropriate sample preparation and dilution, SP-ICP-MS enables NPs to be detected from the elemental signals generated from individual particles as they pass through the plasma. The signal generated by a single NP lasts for about 1 ms, so rapid data analysis software is needed to capture the transient signal. This is done using fast time resolved analysis (TRA) acquisition, which allows single element acquisition at a sampling rate of 100 µs (10,000 measurements per second). With the option of using no settling time between measurements of a particular element, the software can integrate multiple points across the ion plume for each single-particle event, providing sufficient points to define the nanoparticle peak shape (~5 to 10 points/peak). For the SP-ICP-MS analysis of multiple elements, additional rapid acquisition software has been developed that can collect data for up to 16 elements in a single sample, and also optimize the method parameters, thus simplifying the analysis.
Using a rapid multielement NP analysis program, data are collected sequentially for multiple elements, using optimum conditions for the measurement of each individual element. The results for each NP are automatically collated into a single table of results, speeding up the analysis compared to measuring each element individually. Sampling in this manner also prevents agglomeration, or settling of the sample, so a more representative aliquot is analyzed for each analyte under the same analytical conditions. This is demonstrated in Figure 2, which shows a schematic representation of conventional (one element at a time, requiring sample uptake and rinse steps for each element) and rapid multielement NP acquisitions (requiring just one sample uptake and rinse time for all analytes).
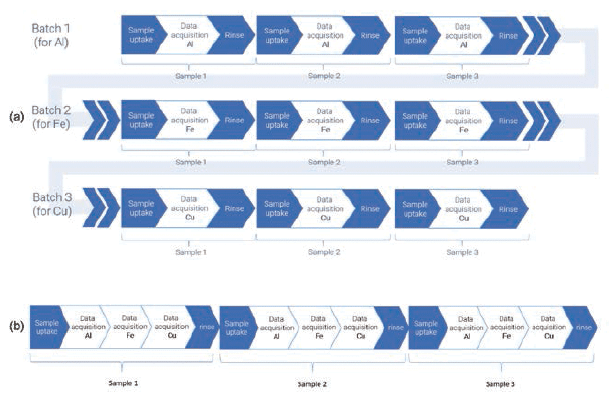
Figure 2: Example of (a) the conventional single element SP-ICP-MS method (top three batches) as compared to (b) a multi-element SP-ICP-MS method (bottom).
Experimental
Reference Materials, Samples, and Sample Preparation
Two platinum (Pt) reference materials from nanoComposix (San Diego, CA, US) were used to confirm the accuracy of the method. The reference materials contained 30 and 50 nm sized particles, and were diluted with deionized water (DIW) to a particle concentration that was calculated to give approximately 200–500 particle counts per min. The solutions were sonicated for ~3 min before analysis, to ensure sample homogeneity.
A stainless steel nanopowder containing multiple nanoparticles was prepared for analysis by placing 41.6 mg of the nanopowder into 10 mL of 0.1% ethanol, and making up to 50 mL with DIW. The solution was sonicated, and then diluted an additional 10,000 times with DIW in a 50 mL vial and 50 µL of ethanol was added to aid suspension.
A sample of moat water and bottled drinking water were sonicated as received for about 3 min. Two separate tap water samples were diluted 100x using DIW before sonication. Various natural waters were collected, including a river estuary sample, a sample collected upstream of a drain, water from a runoff drain, water downstream of the drain, and a seawater sample. These water samples were diluted 1000 times, using DIW before sonication. The reagents as 1% tetramethylammonium hydroxide (TMAH), 1% sulfuric acid (H2SO4), 1% hydrochloric acid (HCl), 1% nitric acid (HNO3), 1% hydrogen peroxide (H2O2), and 1% ammonium hydroxide (NH4OH) were prepared using 0.5 mL of the reagent into 50 mL of DIW. Then, 50 µL of ethanol was added to all samples before analysis, to aid suspension of insoluble NPs in solution.
Instrumentation
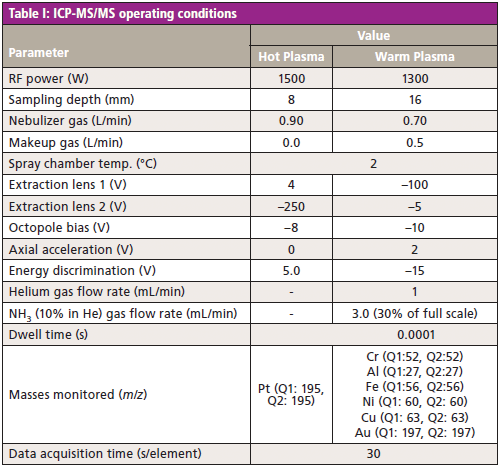
An 8900 Triple Quadrupole ICP-MS instrument (Agilent Technologies) was used for the analysis of NPs in the reference materials, stainless steel nanopowder, environmental samples, and laboratory reagents. The instrument's ion lens system was configured for maximum sensitivity, together with a PFA microflow nebulizer. The samples were delivered via the SPS4 autosampler (Agilent Technologies) through a peristaltic pump. Hot plasma conditions were used initially to confirm the sample preparation method, and to assess the accuracy of the method for the determination of the Pt NP reference materials. Following a successful initial evaluation, the system was further optimized based on the evaluation of a gold (Au) NP reference materials from nanoComposix (San Diego, California). Warm plasma conditions were used as a compromise between high sensitivity and low background for the measurement of Cr, Al, Fe, Ni, Cu, and Au NPs. Instrument operating parameters are given in Table I.
Nebulization Efficiency Measurement
To convert the signals measured using SP-ICP-MS to the particle content of the original sample, it is necessary to calculate the nebulization efficiency, which is the ratio of the amount of analyte entering the plasma to the amount of analyte delivered to the nebulizer. Here, the nebulization efficiency was calculated by measuring a reference material of known particle size. A 60 nm Au NP reference material dispersed in 0.1% ethanol in deionized water and a 1.0 ppb ionic gold solution in (1% nitric acid in 0.5% hydrochloric acid) were measured. The single NP software automatically calculated the nebulization efficiency of 4.9% and 3.2% for the hot and warm plasma conditions, respectively. The time-resolved signal and size distribution graphs of Au NPs in 0.1% ethanol in deionized water are shown in Figure 3.
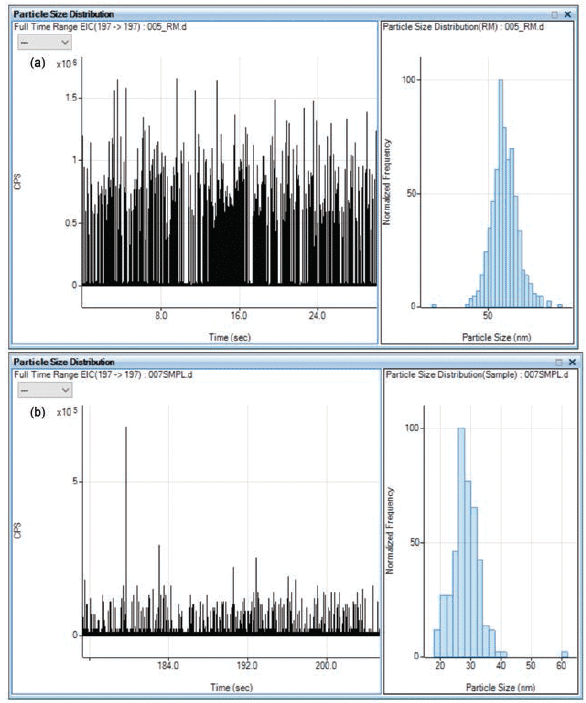
Figure 3: Time-resolved signal and size distribution of (a) 60 nm, and (b) 30 nm gold nanoparticles (Au NPs) in 0.1% ethanol in deionized water solution.
Analysis of Reference Materials
The 50 nm and 30 nm Pt NP reference materials were measured in duplicate, as shown in Figure 4. The respective measured mean size of 48.5 nm and 31 nm and median size of 48.5 nm and 31 nm agreed well with the certificate values of 46 + 5 nm and 30.6 + 3.1 nm obtained by transmission electron microscopy (TEM)-ultraviolet-visible (UV-vis), and dynamic light scattering (DLS).
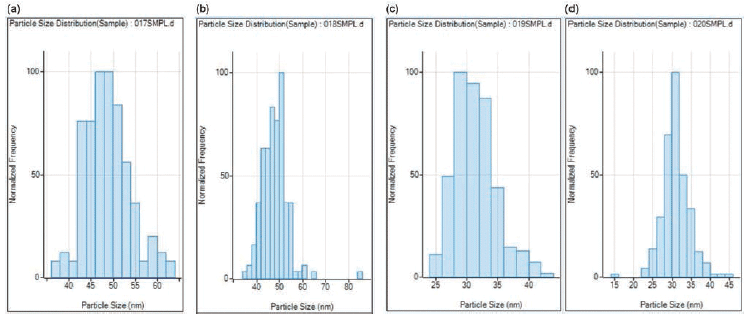
Figure 4: Size distribution results in duplicate of (a, b) 50 nm, and (c, d) 30 nm platinum nanoparticles (Pt NPs) as reference materials.
The 60 and 30 nm Au NP reference materials were also measured in duplicate, as shown in Figure 5. The respective measured 60 nm and 27.5 nm for both the mean and median size agreed well with the certificate values of 60 + 6 nm and 31 + 3 nm obtained by TEM, UV-vis, and DLS.
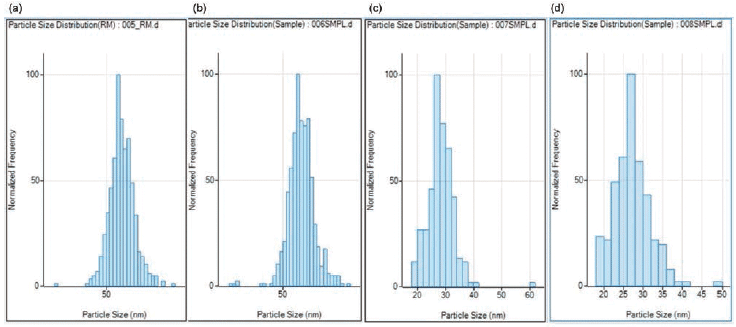
Figure 5: Size distribution results in duplicate of (a, b) 60 nm, and (c, d) 30 nm gold nanoparticles (Au NPs) as reference materials.
Analysis of Multiple Element NPs in Stainless Steel
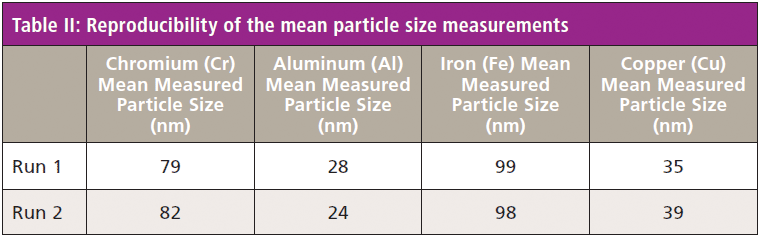
The multielement SP-ICP-MS method was used to analyze a stainless steel nanopowder, to demonstrate that the method could determine multiple NPs in a complex material. The time resolved signal and size distribution for Cr, Al, Fe, and Cu NPs are shown in Figure 6. The results in Table II show that good reproducibility was obtained for the mean and median particle sizes, although some elements showed size variability as the NP's were not all uniform in diameter.
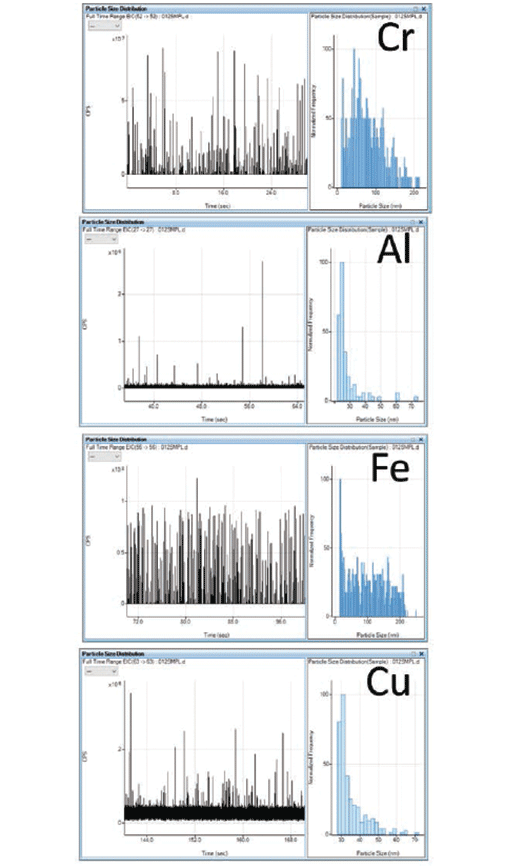
Figure 6: Time-resolved data (left) and particle size distribution (right) for chromium (Cr), aluminum (Al), iron (Fe), and copper (Cu)-containing nanoparticles (NPs) in a stainless steel nanopowder.
Application of the Method to the Analysis of Natural Waters and Chemicals
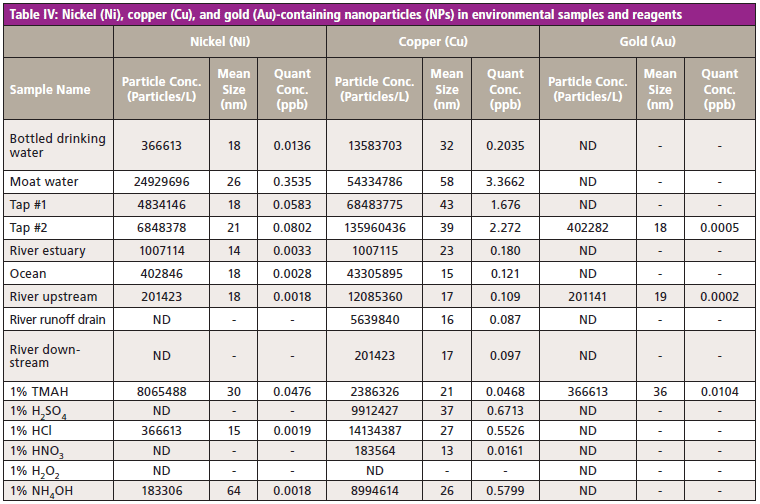
Once a multiple element NP method has been established, it can easily be applied to the analysis of a wide range of samples. As a proof-of-concept, a series of environmental samples including a bottled drinking water, a sample taken from a large pool of water located outside of an office complex, and various river and seawaters, were analyzed using the method, together with six common laboratory reagents. The results given in Tables III and IV show that relatively high levels of Al and Fe-based NPs were present in the environmental samples, especially the moat, river, and sea waters. Relative to this group of samples, the concentration of Cr NPs was highest in the moat water and in tetramethylammonium hydroxide.
Because Fe is a common component of the process equipment used in general manufacturing of chemicals and products, there is an increasing demand to measure Fe-based NPs. The concentration of Fe (and Cr) NPs in tetramethylammonium hydroxide and NH4OH was relatively high compared to the other reagents.
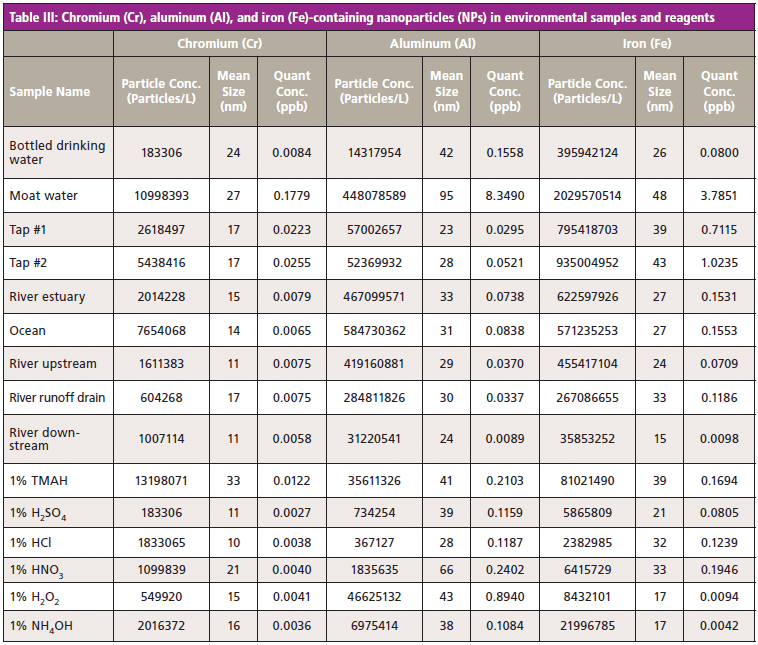
The mean size of the particles ranged from as small as 10 nm up to 95 nm. However, for the analysis of the smallest-sized particles, ICP-MS instrumentation with very high sensitivity and low background is essential to support detection.
Conclusion
The determination of multiple element nanoparticles by SP-ICP-MS is a relatively new application. This study has shown that a triple quadrupole ICP-MS with a double mass selection capability (MS/MS mode) has delivered the high sensitivity, low background, and interference control necessary for the characterization of Cr, Al, Fe, Ni, Cu, Pt, and Au nanoparticles.
The study has also showed that environmental and industrial chemical samples containing nanoparticles composed of different or multiple elements can be determined quickly using SP-ICP-MS/MS. Compared to acquiring data separately for each NP, specially developed rapid acquisition software was used to simplify the analytical method and significantly shorten the sample run times.
Given that regulations are likely to be introduced for nanoparticles across different industry-sectors, ICP-MS is expected to play a central role in the enforcement and development of any new guidelines. Multiple element SP-ICP-MS methods have the potential to more fully characterize the NP content of bulk chemicals, foods, consumer products, medicines, and environmental samples, as well as supporting research projects.
References
(1) F. Laborda, E. Bolea, and J. Jiménez-Lamana, Anal. Chem. 86(5), 2270–2278 (2014)
(2) J. Tuoriniemi, G. Cornelis, and M. Hassellöv, Anal. Chem. 84, 3965-3972 (2012).
(3) C. Degueldre, P.-Y. Favarger, and C. Bitea, Anal. Chim. Acta 518, 137-142 (2004).
(4) E. Bolea-Fernandez, D. Leite, A. Rua-Ibarz, L. Balcaen, M . Aramendía, M. Resano, and F. Vanhaecke, J. Anal. At. Spectrom. 32, 2140–2152 (2017).
(5) E. Bolea-Fernandez, L. Balcaen, M. Resano and F. Vanhaecke, J. Anal. At. Spectrom. 32, 1660–1679 (2017).
L. Craig Jones

L. Craig Jones is an Application Scientist at Agilent, in Santa Clara, California. Craig has been with Agilent since 2004, and has been involved with multiple type of applications for ICP-MS, including environmental, pharmaceutical, nutraceutical, semiconductor, geologic, and clinical analyses, to name a few. Craig obtained a bachelor of science degree in chemistry from Fort Lewis College, in Durango, Colorado.
Mark Kelinske

Mark Kelinske is a Product Specialist at Agilent in Elm Mott, Texas, specializing in advanced ICP-MS and ICP-MS/MS techniques. He received his undergraduate and graduate degrees from Texas A&M University, in College Station, Texas. Prior to Agilent, Mark was a senior research scientist and research group manager with Southern Research Institute, in Birmingham, Alabama, where he focused on low-level analytical chemistry, method development, and research program management.
Emmett Soffey

Emmett Soffey is an ICP-MS Product Specialist at Agilent, in Seattle, Washington. He received his undergraduate degree from Cornell University. Prior to Agilent, he worked at Brookhaven National Labs studying acid rain in the Adirondack Mountains of northern New York, and then went on to work at a number of commercial analytical labs operating GC and GC/MS. Direct correspondence to: emmett_soffey@agilent.com
Robert Thomas

Robert Thomas column editor of "Atomic Perspectives," is the principal of Scientific Solutions, a consulting company that serves the application and writing needs of the trace element user community.

Synthesizing Synthetic Oligonucleotides: An Interview with the CEO of Oligo Factory
February 6th 2024LCGC and Spectroscopy Editor Patrick Lavery spoke with Oligo Factory CEO Chris Boggess about the company’s recently attained compliance with Good Manufacturing Practice (GMP) International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) Expert Working Group (Q7) guidance and its distinction from Research Use Only (RUO) and International Organization for Standardization (ISO) 13485 designations.