Article
Spectroscopy
Spectroscopy
In Situ Raman Measurements of Solid Oxide Fuel Cell Devices
Author(s):
Volume 29, Number 12
Pages 24-30
Solid oxide fuel cells (SOFCs) are versatile electrochemical devices that can operate with a wide variety of carbon containing fuels with high efficiencies. Because of high activation barriers toward fuel cell processes, SOFCs require high operation temperatures (≥650 °C). These high-temperature environments generate significant amounts of blackbody radiation that limit the methods capable of performing in situ characterization of material properties and reaction mechanisms associated with electrochemical fuel oxidation. Vibrational Raman scattering, using a sufficiently short wavelength excitation source, minimizes interference of the blackbody emission and enables experiments to examine carbon accumulation and electrochemical oxidation of SOFC operating at temperature up to 800 °C. The experiments described in this work use in situ Raman spectroscopy and a custom SOFC electrochemical assembly to correlate electrochemical performance of these devices with chemical processes occurring simultaneously at temperatures ≥700 °C when operating with methanol as a fuel source. Specifically, Raman spectroscopy was used to observe the formation of ordered and disordered graphitic deposits on the Ni/YSZ anode. These graphitic deposits were likely responsible for the degradation in electrochemical performance that was observed in voltammetry and impedance measurements. Unlike traditional electrochemical measurements or ex situ analyses, the optical method used in this work provides real-time, materials-specific in situ information about the chemistry occurring in operating SOFCs.
With the current deteriorating state of our environment and an increasing demand on a limited supply of fossil fuels, finding technologies that utilize renewable resources efficiently while producing minimal pollution is a priority for any national energy policy. Of the available alternative energy technologies, solid oxide fuel cells (SOFCs) have attracted considerable attention because of their ability to provide electricity from a variety of fuels (H2, CO, and low-molecular-weight alkanes and alcohols) with efficiencies as great as 80% in combined heating and power applications (1,2). The large activation energy barriers associated with oxide anion diffusion through the electrolyte (100 kJ/mol) and molecular oxygen reduction at the cathode (223 kJ/mol) require that these devices operate at elevated temperatures (600–800 °C) (3,4). These high operational temperatures present research challenges when attempting to perform in situ measurements to investigate the material and chemical processes occurring in these devices during operation. These challenges arise from an excess of blackbody radiation at these elevated temperatures and optically inaccessible device architectures to maintain high operating temperatures (5). The research described in this article illustrates how in situ Raman spectroscopy coupled with an optically accessible SOFC assembly can explore reaction mechanisms associated with a direct methanol fuel breaking down on the SOFC anode surface and its role in electrochemical performance.
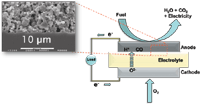
SOFCs rely on the electrochemical oxidation of a fuel to provide an electrical current (1,6–19). In the most general sense, the overall chemistry occurring in SOFCs converts a fuel into products and electricity in the presence of oxide ions that have already been electrochemically reduced (1,6–19). The SOFC device consists of three principal components: an electrolyte and two electrodes (anode and cathode), as shown in Figure 1 (1–3,20). The oxide ions arrive at the anode after diffusing through vacancies in the electrolyte matrix (6,21–28). Oxidation of the fuel is thought to happen at the three-phase boundary (TPB), the region where the ionically conducting electrolyte, the electronically conducting anode, and the gas-phase fuel mixture meet (9,11,29–33). At the TPB, electrons are carried away into the circuit and arrive at the cathode where they reduce the adsorbed oxygen atoms to form O2- (2,6,7,9). An important point to note, however, is that these proposed mechanisms are speculative and are not experimentally validated. The high operating temperatures of SOFCs and inaccessible device architectures make it extremely difficult to validate proposed kinetic models because of lingering questions about the fundamental reactions occurring on the surfaces of SOFC materials.
Because of experimental challenges, electrochemical measurements are typically used to study these SOFC devices (34–37). These techniques can provide information about the resistive elements and electrochemical efficiencies of the SOFC devices (34–37). Conditions such as temperature, cell potential, and fuel identity can be varied to better understand how SOFCs convert reactants into products and generate electricity. Specifically, two methods commonly used to characterize SOFC performance are linear sweep voltammetry (LSV) and electrochemical impedance spectroscopy (EIS) (34–37). LSV is an electrochemical technique that measures a change in cell current at the anode while potential is linear swept between the anode and the cathode of the SOFC (8,38–40). EIS is a technique that measures the frequency-dependent impedance of materials and the results are then modeled with different rate processes assigned to charge and mass transfer processes (34–37). Both of these methods provide valuable information about changes in performance and operating conditions, but the techniques are intrinsically nonspecific and cannot provide detailed information about the structural and chemical changes that are leading to these observed changes in electrochemical performance.
Because of challenges associated with making chemically specific, in situ measurements, ex situ techniques are normally used to support findings from electrochemical measurements. These techniques can also provide valuable information, but since they are conducted after the device has been completely cooled and disassembled, one can only infer how electrochemical performance during operation corresponds to the material changes observed after the fact. Recently, researchers in this field have been exploring the use of in situ methods to explore the fundamental science occurring in these devices. Unlike ex situ methods, these measurements are performed while the device is running or under extreme operating conditions. These techniques include using near-infrared radiation to observe exothermic and endothermic reactions associated with fuel oxidation (41,42), X-ray spectroscopy to monitor changes in oxidation states and crystal lattices of the electrode and electrolyte materials (43,44), and vibrational spectroscopy to monitor material specific changes in the SOFC (44,46). The experiments described in this article use Raman spectroscopy as an in situ technique for studying SOFCs. Specifically, Raman spectroscopy is used to monitor the graphite growth kinetics on SOFC anodes as a function of exposure time to a direct methanol fuel feed. These vibrational measurements are also acquired concurrently with EIS measurements to correlate the effects of graphite growth on fuel cell performance.
Experimental
Solid Oxide Fuel Cell Construction
The electrolyte supported SOFCs described in this article are similar to those that have been used in previous studies (8,45,47,48). These cells consisted of an yttria-stabilized zirconia (YSZ) electrolyte (Tosoh Corporation) with a Ni/YSZ cermet anode and a (La0.8Sr0.2)0.98MnO3-δ (LSM)/YSZ cermet cathode (Fuel Cell Materials Inc.) that were constructed in-house. The YSZ powder used in construction of the electrolyte consisted of 8% Y2O3 (by mole fraction of Y relative to Zr). A 2.0-g sample of YSZ powder was loaded into a 28-mm die and pressed at 20,000 psi for 5 min to produce a YSZ disk that was then sintered at 1500 °C for 1 h in ambient air with a heat up and cool down rate of 1 °C/min. The resulting electrolyte disk had a diameter of 25 mm and a thickness of 0.80 ± 0.02 mm. A tape casting method was used to apply a 30-µm-thick layer of the anode paste, consisting of 50% by weight of Ni to YSZ, to the newly sintered electrolyte. The electrolyte and anode were then sintered again at 1350 °C for 3 h in ambient air with a heat up and cool down rate of 1 °C/min. The same tape casting method was also used to apply a 30-µm-thick layer of the cathode paste, consisting of 50% by weight of (La0.8Sr0.2)0.98MnO3-δ to YSZ. The electrolyte, anode, and cathode were then sintered at 1150 °C for 1 h in ambient air with a heat up and cool down rate of 1 °C/min. Platinum paste (Heraeus) was used to attach platinum mesh (Alfa Aesar) and gold wire (Alfa Aesar) to the cathode as a current collector, while gold paste (Heraeus) was used to attach silver mesh (Alfa Aesar) and gold wire to the anode to act as the anode current collector.
In Situ Raman SOFC Apparatus
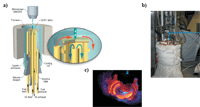
Details regarding the construction of this apparatus have been described previously (8,45,47,48). Alumina paste was used to attach the SOFC membrane–electrode assembly (MEA) atop an alumina tube that was encased inside an optically transparent quartz tube so that in situ Raman measurements could be performed on the anode surface. A network of alumina tubes were used to direct specific gas flows to the anode and cathode. The whole system was surrounded by a split tube furnace to maintain SOFC surface temperatures of 715 °C, while also allowing for optical viewing for the Raman spectrometer. The Raman assembly uses custom-built microscope optics that are used both for excitation and collection of scattered radiation normal to the anode. In the Raman scattering experiments, 488-nm light (8 mW) was focused onto the anode, and backscattered light was collected and sent to the charged couple device (CCD) detector (Renishaw, inVia). Temperatures were maintained by positioning the anode near the top of an open-ended tube furnace and heating the center of the furnace to 1000 °C. In the experiments presented below, vibrational Raman spectra from SOFC anodes were acquired continuously and simultaneously with temporal resolution of 60 s. A schematic of this device and incorporation into the Raman instrument can be seen in Figure 2.
Wavelength Selection
The high operating temperatures of the SOFC device produced a significant amount of blackbody radiation, which is a major challenge that needs to be overcome to conduct in situ characterization measurements (5). Planck's description for blackbody radiation, equation 1, shows that the intensity of the radiation emanating from these SOFCs is directly proportional to operating temperature (5).
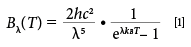
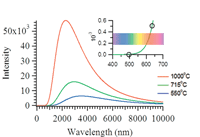
For devices operating at temperatures greater than 700 °C, the majority of the intensity of the blackbody radiation is emitted in the near-infrared region (700–3000 nm) with lower intensities that extend into the mid-infrared region (3000–8000 nm), as shown in Figure 3. Expanding the visible region (380–700 nm), one sees that blackbody radiation also extends down to ~550 nm. Using the single line output of an argon ion laser at 488 nm minimizes the effects of the nonresonant blackbody emission on the Raman spectra backgrounds.
Electrochemical Measurements
All electrochemical measurements were performed using a Princeton Applied Research VMC-2 potentiostat/galvanostat/FRA. To observe the changes in electrochemical performance when using the methanol as a fuel, EIS measurements were performed continuously and concurrently with in situ Raman spectroscopic measurements. Methanol was introduced into the SOFC by bubbling 100 sccm of argon through methanol at 21 °C producing ~13 sccm methanol fuel feed. The chemistry and performance of the SOFC were studied at different cell polarizations, including open circuit voltage (OCV) and 0.30 V. During impedance measurements, the potentiostat polarized the SOFC to well-defined overpotentials with respect to OCV. Here, overpotential (VOP) refers to the difference between OCV and the voltage across the operating cell (VOP = Vcell × VOCV). The EIS measurements were run with an AC amplitude of ±10 mV from a frequency range of 100 kHz to 0.01 Hz.
Results and Discussion
Electrochemical measurements were used to explore the performance of these SOFC devices as a function of methanol fuel exposure. Figure 4 shows the electrochemical data that were collected from the SOFCs exposed to methanol vapor (in an argon carrier) for 30 min. Except for when voltages were changing during LSV measurements, the devices were held at open circuit voltage. The electrochemical data showed that after 30 min of fuel exposure the SOFC had suffered a ~15% reduction in power, Figure 4a. EIS data from the same cell also showed significant changes in their impedance spectra as a function of fuel exposure, Figure 4b. On the basis of previous studies (49,50), the EIS data in Figure 4b were fit to an equivalent circuit containing four elements. The bulk resistance (Rbulk) refers simply to the pure ohmic resistance associated with oxide diffusion through the electrolyte. The other three elements are RC circuits and the frequency dependence of these three elements is assigned to different processes occurring in the working SOFC (49,50). The high-frequency feature in the EIS data at low impedance is assigned to cathodic processes. The middle arc is associated with charge transfer activity at the anode. The highest impedance arc, at low frequency, reflects mass transport processes at the anode. The total polarization resistance, defined as the impedance between the two x-intercepts, grows significantly as a function of exposure to methanol. The single quantity most responsible for the changing impedance spectra is the magnitude of the middle arc, the intermediate frequency arc assigned to changes in the chemistry occurring at the SOFC anode.
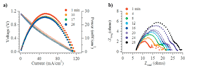
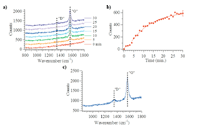
While electrochemical methods can explore the performance of the SOFC as a function of fuel exposure, these techniques cannot identify with material or molecular specificity of the species present. Optical spectroscopy, vibrational in particular, is uniquely well suited for in situ observation of specific molecular and material species. Figure 5 shows a sequence of vibrational Raman spectra that indicate the relative amounts of carbon (graphitic) on Ni/YSZ cermet anodes held at OCV and exposed to a direct methanol fuel feed. Several observations stand out. First, the strongest feature observed in both series of spectra is a sharp band at ~1560 cm-1. This feature is associated with the "G" band of graphite in the literature and is characteristic of highly ordered, sp2 hybridized, graphite having very few defects or grain boundaries (45). The fact that this feature appears provides definitive evidence that methanol is capable of forming carbon deposits on the Ni/YSZ cermet anodes and that these carbon deposits are likely responsible for the changes that were observed in the electrochemical measurements. Figure 5b plots the intensity of the 1560 cm-1 band as a function of fuel exposure in 1 min intervals. Graphite growth appears to be self-limiting as the growth of the "G" band approaches an asymptotic limit after ~25 min. A closer look at the graphite growth kinetics, for methanol as a fuel, reveals that it follows a two-step mechanism; one step occurring from 0 to 5 min of earlier fuel exposure and then a more aggressive growth from 5 to 30 min. Starting at ~15 min, Raman spectra (shown in Figure 5a) begin to show a second feature growing in at ~1340 cm-1. In Figure 5c, higher sensitivity spectra with longer acquisition times verified the presence of this small feature. This feature is assigned to the graphite "D" band and is attributed to disordered carbon with a large number in site defects, grain boundaries, and sp3 hybridization. Carbon with sp3 hybridization allows disordered graphite to form in nonplanar sheets as compared to the extended planar sheets of the sp2 carbon that gives rise to ordered "G" graphite. We speculate that the emergence of the disordered graphite could be responsible for the second growth mechanism seen in Figure 5b.
To verify the correlation between the electrochemical and the in situ Raman measurements, polarization studies were performed to look at the effect that changes in current and voltage would have on the impedance and in situ spectroscopic measurements of these devices (Figure 6). Polarizing the SOFC increases the current being drawn across the device, which drives the fuel oxidation mechanisms in the forward direction as a result of an increase in the oxide anion flux through the electrolyte to the anode. Under these conditions, the increase in oxide anion concentration at the anode might aid in the oxidation of unwanted graphite that is present on the anode. In situ Raman data shown in Figure 6a independently confirms this assertion by observing the "G" band graphite growth kinetics for the SOFC operated at OCV and polarized to 0.30 V. The kinetic traces show a clear reduction in the total amount of graphite formed as the cell is subjected to the higher overpotential. Graphite formed from methanol shows a threefold reduction in the 1560 cm-1 feature at 0.30 V polarization as compared to OCV. A direct correlation between the electrochemical and spectroscopic measurements is observed when concurrent EIS measurements are performed with the in situ Raman measurements, Figure 6b. There is an initial increase in capacitance and resistance of the device from 1 to 4 min, followed by a minimal increase in these resistive elements as fuel exposure continues from 5 to 30 min. In Figure 6a, it is also observed that there is an initial fast graphite growth mechanism from 1 to 4 min that seems to slow after 4 min. Comparing the EIS to the Raman data for these polarization studies, it is observed that there is a direct correlation to graphite growth and electrochemical performance.
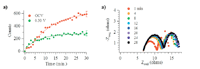
Conclusions
The combination of electrochemical techniques and vibrational Raman spectroscopy has been used to measure performance and in situ growth of carbon deposits on SOFC Ni/YSZ anodes at 715 °C operating with a direct methanol fuel feed. The two techniques, electrochemical and in situ spectroscopy, used together construct a more complete picture of the chemistry occurring on SOFC anodes than either method can do on its own. In situ Raman data identify the carbon deposits as graphitic with deposits formed from methanol being highly ordered with a small degree of disorder. The Raman data can be analyzed further to quantify the growth kinetics of these carbon deposits. Graphite appeared to form from multiple pathways given the rapid onset of additional intensity in the Raman spectra acquired after ~5–7 min. Although carbon deposition from this fuel is not as detrimental to the anode as other higher-weight fuels previously studied (48), the deposits do lead to decreased performance as determined from electrochemical experiments. These results imply that devices intended to operate with methanol should use steps to ensure that the fuel has undergone sufficient preprocessing to inhibit carbon growth. Such steps can include operating at high overpotentials, external or internal reforming, or operating at higher temperatures.
Unlike traditional standalone electrochemical measurements or ex situ analyses, the optical method used in this work provides real time, materials-specific in situ information about the chemistry occurring in operating SOFCs. Such data provide the quantitative benchmarks needed to test proposed models of electrochemical fuel oxidation in these devices. More importantly, being able to differentiate mechanisms of SOFC operation as a function of fuel identity and cell polarization can help guide the development of new, more effective SOFC materials and architectures.
Acknowledgments
The authors gratefully acknowledge support from the United States Office of Naval Research (Grant Numbers N00014-10-WX-1-0954 and N00014-12-WX02-0412) and support for instrumenation from the Murdock Charitable Trust (Grant Number 2010085).
Bryan C. Eigenbrodt, PhD, is an assistant professor in the chemistry department at Villanova University in Villanova, Pennsylvania. Robert A. Walker, PhD, is a professor in the Department of Chemistry and Biochemistry and the director of the Materials Science Program at Montana State University in Bozeman, Montana. Direct correspondence to: bryan.eigenbrodt@villanova.edu
References
(1) R. Ormerod, Chem. Soc. Rev.32, 17–28 (2003).
(2) C. Song, Catal. Today77, 17–49 (2002).
(3) J. Goodenough, Annu. Rev. Mater. Res. 33, 91–128 (2003).
(4) R. Devanathan, W. Weber, S. Singhal, and J. Gale, Solid State Ionics177, 1251–1258 (2006).
(5) T. Merritt and F. Hall, Proc. Inst. Rad. Eng.47, 1435–1441 (1959).
(6) M. Pomfret, C. Stoltz, B. Varughese, and R. Walker, Anal. Chem.77, 1791–1795 (2005).
(7) H. Zhu, R. Kee, V. Janardhanan, O. Deutschmann, and D. Goodwin, J. Electrochem. Soc.152, A2427–A2440 (2005).
(8) M. Pomfret, J. Owrutsky, and R. Walker, Anal. Chem. 79, 2367–2372 (2007).
(9) J. Nielsen and T. Jocobsen, Solid State Ionics178, 1769–1776 (2008).
(10) R. Bove and S. Ubertini, J. Power Sources159, 543–559 (2006).
(11) H. Funkunaga, M. Ihara, K Sakaki, and K Yamada, Solid State Ionics86, 1179–1184 (1996).
(12) J. Nam and D. Jeon, Electrochim Acta.51, 3446–3460 (2006).
(13) B. Feng, C. Wang, and B. Zhu, Electrochem. Solid State Lett.9, A80–A81 (2006).
(14) D. Hirabayashi, A. Hasimoto, T. Hibino, U. Harda, and M. Sano, Electrochem. Solid State Lett.7, A108–A110 (2004).
(15) M. Ihara and S. Hasegawa, J. Electrochem. Soc. 153, A1544–1546 (2006).
(16) Y. Lin, Z. Zhan, J. Liu, and S. Barnett, Solid State Ionics176, 1827–1835 (2005).
(17) Y. Nabae, I. Yamanaka, M. Hatano, and K. Otsuka, J. Electrochem. Soc.153, A140–A145 (2006).
(18) A. Sin, E. Kopin, Y. Dubitsky, A. Zaopo, A. Arico, D. La Rosa, and V Antonucci, J. Power Sources 164, 300–305 (2007).
(19) K. Walters, A. Dean, H. Zhu, and R. Kee, J. Power Sources123, 182–189 (2003).
(20) N. Minh, Solid State Ionics174, 271–277 (2004).
(21) A. Feinberg and C. Perry, J. Phys. Chem. Solids42, 513–518 (1981).
(22) C. Perry, A. Fienberg, and R. Currat, Bull. Amer. Phys. Soc.26, 405-411 (1981).
(23) C. Perry and A. Fienberg, Solid State Commun. 36, 519–522 (1980).
(24) K. Swider and W. Worrell, J. Electrochem. Soc.143, 3704–3706 (1996).
(25) N. Sammes and Z. Cai, Solid State Ionics 100, 39–44 (1997).
(26) J. Lee, S. Yoon, B. Kim, J. Kim, H. Lee, and H. Song, Solid State Ionics 144, 175–184 (1997).
(27) P. Vernoux, M. Guth, and X. Li, Electrochem. Solid State Lett.12, E9–E11 (2009).
(28) A. Svensson, S. Sunde, and K. Nisancioglu, J. Electrochem. Soc.145, 1390–1400 (1998).
(29) A. Martinez and J. Brouwer, J. Power Sources195, 7268–7277 (2010).
(30) B. Kenney, M. Valdmanis, C. Baker, J. Pharoah, and K. Karan, J. Power Sources189, 1051–1059 (2009).
(31) V. Janardhanan, V. Heuveline, and O. Deutschmann, J. Power Sources178, 368–372 (2008).
(32) W. Zhu, D. Ding, and C. Xia, Electrochem. Solid State Lett.11, B83–B86 (2008).
(33) M. Schmidt, K. Hansen, K. Norrman, and M. Mogensen, Solid State Ionics 180, 431–438 (2009).
(34) A. Bieberle, L. Meier, and L. Gauckler, J. Electrochem. Soc.148, A646–A656 (2001).
(35) S. Bebelis and S. Neophytides, Solid State Ionics447, 152–153 (2002).
(36) A. Bieberle, L. Meier, and L. Gauckler, Solid State Ionics146, 23–41 (2002).
(37) S. Sundes, Electrochim. Acta 42, 2637–2648 (1997).
(38) R. Simpson, Introductory Electronics for Scientist and Engineers (Allyn and Bacon, Inc., Pearsoneducation, London, UK, 1987).
(39) J. Wang, Analytical Electrochemistry (John Wiley & Sons, Hoboken, New Jersey, 2006).
(40) A. Bard and L. Faulkner, Electrochemical Methods: Fundamentals and Applications (John Wiley & Sons, New York, New York, 2001).
(41) M. Pomfret, D. Steinhurst, D. Kidwell, and J. Owrutsky, J. Power Sources195, 257–262 (2010).
(42) M. Pomfret, D. Steinhurst, D. Kidwell, and J. Owrutsky, J. Electrochem. Soc. Trans.25, 839–845 (2009).
(43) Y. Orikasa, E. Crumlin, and S. Sako, Electchem. Soc. Electrochem Lett.3, F23–F26 (2014).
(44) R. Woolley, M. Ryan, and S. Skinner, Fuel Cells13, 1080–1087 (2013).
(45) B. Eigenbrodt, M. Pomfret, D. Steinhurst, J. Owrutsky, and R. Walker, J. Phys. Chem. C. 115, 2895–2903 (2011).
(46) B. Eigenbrodt and R. Walker, Anal. Meth.3, 1478–1484 (2011).
(47) M. Pomfret, J. Marda, G. Jackson, B. Eichhorn, A. Dean, and R. Walker, J. Phys. Chem. C. 112, 5232–5240 (2008).
(48) M. Pomfret, J. Owrutsky, and R. Walker, J. Phys. Chem. B.110, 17305–17308 (2006).
(49) P. Hofmann and K. Panopoulos, J. Power Sources195, 5320–5339 (2010).
(50) A. Bieberle and L. Gauckler, Solid State Ionics135, 337–345 (2000).

Newsletter
Get essential updates on the latest spectroscopy technologies, regulatory standards, and best practices—subscribe today to Spectroscopy.





