Optical Molecular Spectroscopy in Combination with Artificial Intelligence for Process Analytical Technology
Spectroscopy
In celebration of Spectroscopy’s 35th Anniversary, leading experts discuss important issues and challenges in analytical spectroscopy.
This article highlights the use of optical molecular spectroscopy in combination with artificial intelligence (AI) methods in the framework of process analytical technology (PAT). Specifically, we focus on the combination of Raman, infrared (IR), mid-IR, and near-infrared (NIR) spectroscopy and AI methods such as machine learning, deep learning, and chemometrics for PAT. The combination of these molecular spectroscopy methods in combination with tailor-made AI data analysis methods has the potential to revolutionize PAT in the upcoming years.
Introduction
Innovations in quality assurance and process control are of essential importance for the coming decades given the immensely growing world population, the development of demography, and the increasing demands of people on products and services. Quality assurance is subject to constantly increasing requirements and is essential for every individual as well as for companies for economic reasons. As a result of industrial processes one expects to see high safety, high and stable product quality, high yields, low consumption of resources, reduced influence of the variability of raw materials as well as long product durability. In recent years, new developments in the field of process analytics have been continuously observed.
The quality by design (QbD) concept plays a decisive role in this development (1). This concept is based on the fact that a high-quality level of end products cannot be achieved by testing the products alone, but by an intelligent design of the entire manufacturing process into which the products are incorporated. This approach requires a comprehensive understanding of the entire manufacturing process. If one is informed about all relevant characteristics along a production process, one gets a better picture of the quality of a product or intermediate product than by certifying the final product- which is still generally accepted today as a measure of quality. The technology that makes QbD possible is process analytical technology (PAT). PAT is an approach for the improved control of production processes. Although originally promoted by the U.S. Food and Drug Administration (FDA) for use in the pharmaceutical sector, the PAT initiative is also gaining in importance for related industries, such as the food industry. If PAT is moved further forward along the entire production process, deviations from specified guideline values and possible risks in production and manufacturing processes can be detected and eliminated immediately.
PAT distinguishes between off-line, at-line, on-line and in-line measurement methods (1). In off-line measurements, samples are taken from the process and analyzed in a laboratory environment that is clearly separated from the industrial plants. Off-line analysis therefore always shows significant delay times between the detection of irregularities and the counteraction. In at-line measurements, the sample is taken from the process stream and analyzed with analytical equipment located in the immediate vicinity of the industrial plants. This process significantly reduces the reaction time for countermeasures. In on-line measurements, the samples are not completely removed from the process stream, but are temporarily separated, such as via a bypass system that transports the sample directly through the on-line measuring device, where the sample is analyzed in the immediate vicinity of the industrial processing and then recombined with the process stream. When using in-line instruments, the sensor is immersed directly into the process stream and remains in direct contact with the unaltered material stream. Increasingly, the need for in-line process analytics is growing and with it the availability of appropriate measurement technologies. From the large variety of analytical methods, optical molecular spectroscopy is one of the most promising approaches, which can best be integrated in-situ within an in-line process control.
PAT Methods
In order to be able to intervene quickly in the event of changes in the process that lead to a reduction in the quality of the end product, powerful in-situ analytical approaches are required. In this context, optical spectroscopic methods in particular are becoming increasingly important for in-line process monitoring. Optical spectroscopic methods are usually nondestructive and provide continuous information that can be used for process control. Therefore, optical spectroscopic methods are becoming more and more important in PAT. Depending on the measurement problem, a wide range of wavelength ranges and phenomena of light-matter interaction can be used, such as:
- ultraviolet-visible (UV-vis) spectroscopy (electronic excitation)
- fluorescence spectroscopy (absorption and emission of electrons)
- vibrational spectroscopy (NIR or mid-IR absorption and Raman spectroscopy)
- terahertz spectroscopy
- laser induced breakdown spectroscopy (LIBS).
All these spectroscopic techniques differ in molecular selectivity, sensitivity, readiness for use in PAT as a result of special sampling conditions, and so on. Vibrational spectroscopic methods are characterized by a particularly high molecular selectivity, because they provide direct information about characteristic molecular vibrations and thus about the molecular structure. A vibrational spectrum can therefore be seen as a kind of characteristic “molecular fingerprint” of a molecular species, which enables the identification of organic, inorganic, or biological components. Absorption in the mid-IR range excites fundamental vibrations that can be directly assigned to the basic building blocks in a molecule. NIR spectroscopy, on the other hand, measures vibrational overtones and combination bands and is therefore less selective (no easy direct interpretability) and also less sensitive as compared to mid-IR absorption spectroscopy. Despite these disadvantages, the main advantage of NIR spectroscopy compared to mid-IR spectroscopy is that no sample preparation (such as dilution) is required even at higher concentrations. It is important to emphasize that both NIR and mid-IR spectroscopy are very sensitive to water absorption, which limits a broad applicability of these methods in aqueous environments, such as for the investigation of fermentation processes. For the analysis of aqueous systems Raman spectroscopy can be advantageous compared to NIR and mid-IR spectroscopy. In recent years the potential of Raman spectroscopic methods for PAT has been increasingly recognized (1–3). The advantages of Raman spectroscopy are an unprecedented high specificity and a variety of applications. Raman spectroscopy is a noninvasive technique where no special sample preparation is required. Solid, liquid, and gaseous samples as well as transparent and nontransparent samples or samples with inhomogeneous surface properties can be measured. Limitations to the applicability of Raman spectroscopy result from the possible occurrence of an interfering fluorescence background and the low signal yields in comparison with, for example, mid-IR or NIR absorption or fluorescence spectroscopy. In recent years, however, decisive technical progress has been made to overcome these limitations, in particular through the realization of highly efficient spectrographs and the implementation of novel detector technologies. These advances have led to a significant reduction in acquisition times down to the ms range (4,5).
Implementation
In recent years, new developments in the field of PAT have been continuously observed using optical spectroscopic methods. Especially in the field of chemical and pharmaceutical analysis, optical and spectroscopic methods are used in on-line process monitoring (2,3,6). It can be stated that especially the interest in NIR and Raman spectroscopy-given their potential for fast and nondestructive analysis of samples in different physical states (liquids, solids, pastes, and so on) and their capability to be used on-line or in-line-as potential PAT analysis methods has increased significantly. Although NIR spectroscopy is still predominant in the pharmaceutical industry, Raman spectroscopy is becoming more widespread and has already proven its feasibility for the analysis of critical quality characteristics throughout the drug manufacturing process (2,3).
Worldwide, the demand for high-quality and safety in the food supply are also constantly increasing. Thus, a safe and high-quality food supply can only be guaranteed if new possibilities in analytics and holistic process control are generated. However, food analysis presents PAT with extreme challenges. In comparison to the chemical and pharmaceutical industries, in the field of the food industry, the sample compositions are much more complex. They are mixtures of different substance classes.
Furthermore, the materials are often soft, variable in size, brittle, or slippery. They are extremely susceptible to changes in pressure, temperature, and humidity, making noninvasive, fast methods for process analysis essential. In recent years, technological developments have already been made that make it possible to replace laboratory-based methods with on-line process control. These are mainly based on fluorescence or IR or NIR spectroscopy (7–9). Furthermore, the potential of Raman spectroscopic methods has been increasingly recognized. This potential has been successfully demonstrated using the example of determining the content of glucose, fructose, and sucrose in commercially available soft drinks (10). In the field of drinking water monitoring, Raman-based methods for the in-line detection of smallest amounts of chemicals and pharmaceutical impurities were applied (11).
Furthermore, surface-enhanced Raman spectroscopy (SERS) was applied for the detection of prohibited dyes as well as azo dyes in food (12,13). These representative examples show that optical spectroscopic methods and in particular Raman spectroscopy have enormous potential for on-line process analysis in food production.
To extend the analytical potential with respect to selectivity, sensitivity, and so on, it can be very advantageous to combine several of the above mentioned optical spectroscopic contrast mechanisms in a multimodal approach (14). From a practical point of view, besides the need for high sensitivity and selectivity, the price-to-performance ratio and the simplicity of application of optical spectroscopic methods are of particular importance. The availability of compact, high-performance spectrometers including detectors as well as easy-to-operate compact light sources-and especially lasers with acceptable operating times-play a decisive role.
An essential feature of optical process spectrometers is the coupling of probes or the use of flow cells. Using fiber-optic coupled probes, it is possible to spatially separate spectrometer and application in the process and thus to look directly into individual processes. Fiber-optical sensors help to record data at critical points. As the “sensory organs of process control technology,” they enable quality-optimized automation of processes. In this context, particularly great progress has been made in recent years in the development of high-performance, tailor-made Raman probe concepts, mainly for biomedical applications (15–17), which are also of high relevance for in-line process analysis. In summary, technological developments have been made in the last few years in the areas of light sources, spectrometers, detectors and fiber probes, which will make it possible to replace laboratory-based methods with on-line process control in the future.
Essential challenges for a continuous quality and safety monitoring of the processes as well as the resulting products and materials are seen in the still missing automation of process analytical techniques and here especially in the automated analysis of the recorded spectral measurement data. This automated analysis of the spectral data is accomplished by data analysis pipelines, which combine standardization and correction procedures with chemometric techniques and machine learning models (18,19). The overall aim of these data pipelines is the translation of the optical or spectral data into meaningful higher information (see Figure 1). In PAT this higher information might by the concentrations of given substances, the quantification of the sample composition or the prediction of a sample property like “good”or “bad.”
FIGURE 1: Visualization of Raman spectroscopy as PAT technology: The experimentally recorded Raman spectra from a sample is translated into meaningful higher information using computerized machine learning and chemometrics approaches.
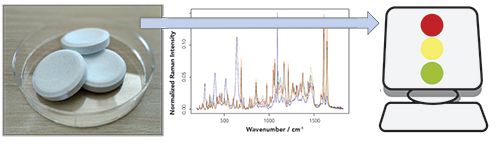
To perform this translation of the experimentally recorded spectral data, standardization and correction procedures are utilized first. For Raman spectroscopy a de-spiking (20), a wavenumber, and spectrometer calibration (21), a background correction (22) and a normalization are performed. These sequential steps correct for corrupting effects and standardize the Raman data, while similar preprocessing steps are done for other optical technologies like IR or NIR spectroscopy. After the data is standardized, machine learning models such as deep learning (23) or chemometric models (19) are utilized for the translation of the preprocessed data into meaningful information. As mentioned above, this higher information might be concentration estimates of substances or compounds of the sample or prediction of sample attributes like “good” and “bad.” Depending on the choice of higher lever information, either regression or classification models are utilized. Most often for spectral measurements, chemometric techniques are utilized whereas for image-based measurements, deep learning methods, like convolutional neuronal networks, are chosen for the analysis. All of these models are optimized based on training data and afterward the models are evaluated for their generalization performance (24). Thereafter, the models can be used for the prediction of unknown test data (with the estimated generalization error) and the prediction can be utilized for process analytics in chemical, pharmaceutical, and food industry.
Conclusions
This contribution provides a short overview on the use of optical molecular spectroscopy such as Raman, IR, mid-IR, NIR spectroscopy as process analytical technology (PAT). An essential step to successfully apply these measurement methods as PAT is their combination with artificial intelligence methods like machine learning, deep learning, and chemometrics in order to translate the spectroscopic information into meaningful higher information such as quantification of the sample composition or the prediction of a sample property. This combination is done by tailor-made data pipelines and is briefly explained for the example of Raman spectroscopy. Overall, this contribution highlights the great potential of optical molecular spectroscopy in combination with artificial intelligence for PAT.
Acknowledgment
Financial support of the EU, the ”Thüringer Ministerium für Wirtschaft, Wissenschaft und Digitale Gesellschaft,” the ”Thüringer Aufbaubank”, the Federal Ministry of Education and Research, Germany (BMBF), the German Science Foundation, the Fonds der Chemischen Industrie, and the Carl-Zeiss Foundation are greatly acknowledged.
References
- Kandelbauer, M. Ruhe, and R. W. Kessler, in J. Popp, V.V. Tuchin, A. Chiou, and S.H. Heinemann, Eds., Handbook of Biophotonics Vol. 3: Photonics in Pharmaceutics, Bioanalysis and Environmental Research (Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 2011), pp. 3–70.
- K.A. Esmonde-White, M. Cuellar, C. Uepmann, B. Lenain, and I.R. Lewis, Anal. Bioanal. Chem.409, 637–649 (2017).
- Nagy, A. Farkas, E. Borbás, P. Vass, Z.K. Nagy, and G. Marosi, AAPS PharmaSciTech. 20, 1 (2019).
- Krafft, I.W. Schie, T. Meyer, M. Schmitt, and J. Popp, Chem. Soc. Rev. 45, 1819–1849 (2016).
- Schie, J. Rüger, A.S. Mondol, A. Ramoji, U. Neugebauer, C. Krafft, and J. Popp, Anal. Chem. 90, 2023–2030 (2018).
- K.A. Bakeev, Ed., Process Analytical Technology: Spectroscopic Tools and Implementation Strategies for the Chemical and Pharmaceutical Industries (Wiley, Hoboken, New Jersey, 2nd Ed., 2010).
- B. Grote, T. Zense, and B. Hitzmann, Food Control 38, 8–18 (2014).
- Niemöller and D. Behmer, in J. Irudayaraj and C. Reh, Eds., Nondestructive Testing of Food Quality (Wiley Blackwell, Ames, Iowa, 2008), Chapter 4.
- Hitzmann, R. Hauselmann, A. Niemoeller, D. Sangi, J. Traenkle, and J. Glassey, Biotechnol. J. 10, 1095–1100 (2015).
- K. Ilaslana, I. Hakki Boyaci, and A. Top, Food Control 48, 56–61 (2015).
- Z. Li, M.J. Deen, S. Kumar, and P.R. Selvaganapathy, Sensors14, 17275–17303 (2014).
- S. Hea, W. Xiea, W. Zhanga, L. Zhang, Y. Wang, X. Liu, Y. Liu, and C. Du, Spectrochim Acta A Mol. Biomol Spectrosc. 137, 1092–1099 (2015).
- V. Peksa, M. Jahn, L. Štolcová, V. Schulz, J. Proška, M. Procházka, K. Weber, D. Cialla-May, and J. Popp, Anal. Chem. 87, 2840−2844 (2015).
- T. Meyer, M. Schmitt, B. Dietzek, and J. Popp, J. Biophotonics 6, 887–904 (2013).
- Krafft, S. Dochow, I. Latka, B. Dietzek, and J. Popp, Biomed. Spectrosc. Imaging 1, 39–55 (2012).
- Latka, S. Dochow, C. Krafft, B. Dietzek, and J. Popp, Laser Photonics Rev. 7, 698–731 (2013).
- Cordero, I. Latka, C. Matthäus, I. W. Schie, and J. Popp, J. Biomed. Opt. 23, 071210 (2018).
- Ryabchykov, S. Guo, and T. Bocklitz, in J. Popp, Ed., Micro-Raman Spectroscopy Theory and Application (De Gruyter, Berlin, Germany, 2018).
- S. Guo, O. Ryabchykov, N. Ali, R. Houhou, and T. Bocklitz, in Comprehensive Chemometrics Molecular Sciences and Chemical Engineering (Elsevier, New York, New York, 2020).
- Ryabchykov, T. Bocklitz, A. Ramoji, U. Neugebauer, M. Foester, C. Kroegel, M. Bauer, M. Kiehntopf, and J. Popp, Chemom. Intell. Lab. Syst. 155, 1–6 (2016).
- T. Bocklitz, T. Dörfer, R. Heinke, M. Schmitt, and J. Popp, Spectrochim. Acta, Part A 05, 544–549 (2015).
- S. Guo, T. Bocklitz, and J. Popp, Analyst 141, 2396–2404 (2016).
- Pradhan, S. Guo, O. Ryabchykov, J. Popp, and T. W. Bocklitz, J. Biophotonics (2020). doi:10.1002/jbio.201960186.
- Guo, T. Bocklitz, U. Neugebauer, and J. Popp, Anal. Methods 9, 4410–4417 (2017).
Thomas Bocklitz is the head of the Research Department of Photonic Data Science, at the Leibniz Institute of Photonic Technology, in Jena, Germany. Michael Schmitt is an assistant professor at the Institute of Physical Chemistry and Abbe Center of Photonics at Friedrich Schiller University Jena, Germany.

Juergen Popp is the scientific director of the Leibniz Institute of Photonic Technology, Jena, Germany, and also holds the chair for Physical Chemsitry at the Friedrich Schiller University Jena, Germany. Direct correspondence to: juergen.popp@ipht-jena.de
