- Spectroscopy-06-01-2011
- Volume 26
- Issue 6
Market Profile: Process FT-NIR for PAT in Pharma and Biopharma
Fourier-transform near-infrared (FT-NIR) spectroscopy continues to be a rapidly growing process analytical technique, particularly in the pharmaceutical and biopharmaceutical industry. The technique offers a number of advantages for online applications, and most of the major NIR instrument vendors now compete in this segment of the market.
Fourier-transform near-infrared (FT-NIR) spectroscopy continues to be a rapidly growing process analytical technique, particularly in the pharmaceutical and biopharmaceutical industry. The technique offers a number of advantages for online applications, and most of the major NIR instrument vendors now compete in this segment of the market.
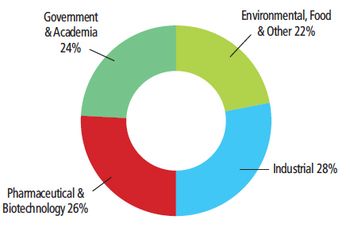
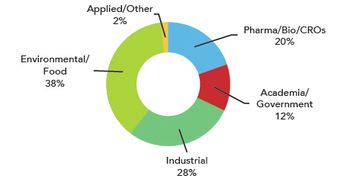
Biopharmaceutical and pharmaceutical process FT-NIR vendor share in 2010.
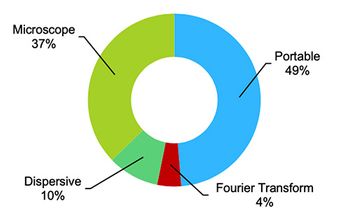
FT-NIR spectrometers make use of a simpler mechanical design than dispersive NIR instruments, and therefore provide a more rugged and reliable design that is advantageous in any industrial setting, including pharmaceutical and biopharmaceutical manufacturing. In addition, FT-NIR provides simultaneous analysis of all frequencies in the spectrum range, rather than scanning individual wavelengths. FT-NIR is also capable of much higher resolution than dispersive instruments, which is important in the pharmaceutical and biopharmaceutical industry.
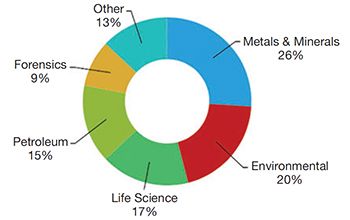
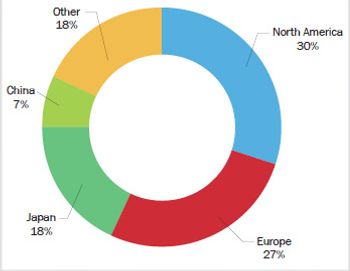
FT-NIR is becoming increasingly popular for process analytical technology (PAT) and other online applications in the pharmaceutical and biopharmaceutical industry, such as monitoring drying and blending processes. The global market for process FT-NIR in the pharmaceutical and biopharmaceutical industry in 2010 was more than $21 million, and it is expected to continue to see annual growth in the mid-teens for the foreseeable future. At least a half dozen instrument vendors are significant competitors in the market.
The foregoing data were extracted from SDi's market analysis and perspectives report entitled Biotech & Pharmaceutical Process Analysis: PAT Instrumentation and More, March 2011. For more information, contact Stuart Press, Vice President, Strategic Directions International, Inc., 6242 Westchester Parkway, Suite 100, Los Angeles, CA 90045, (310) 641-4982, fax: (310) 641-8851,
Articles in this issue
over 14 years ago
Short Coursesover 14 years ago
Maxwell's Equations, Part IIover 14 years ago
Productsover 14 years ago
Classical Least Squares, Part VI: Spectral Resultsover 14 years ago
Vol 26 No 6 Spectroscopy June 2011 Regular Issue PDFNewsletter
Get essential updates on the latest spectroscopy technologies, regulatory standards, and best practices—subscribe today to Spectroscopy.