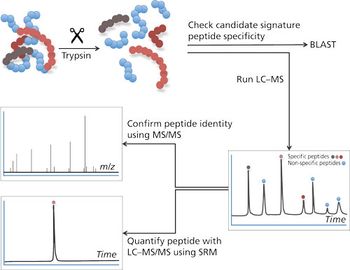
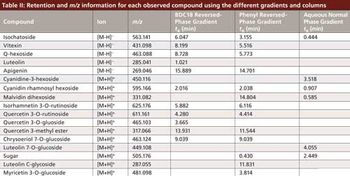
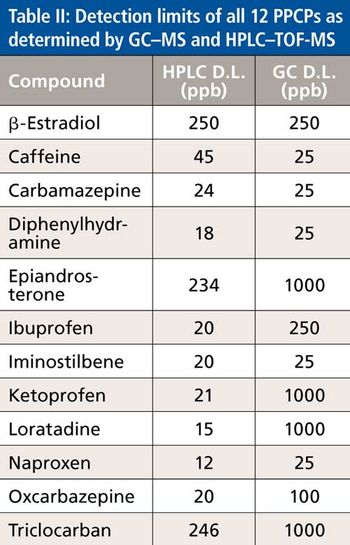
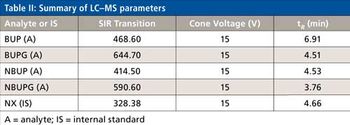
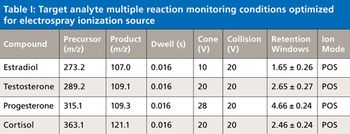
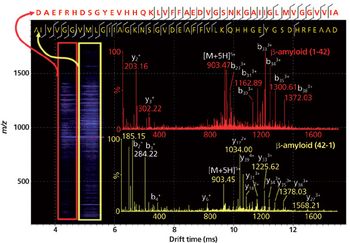
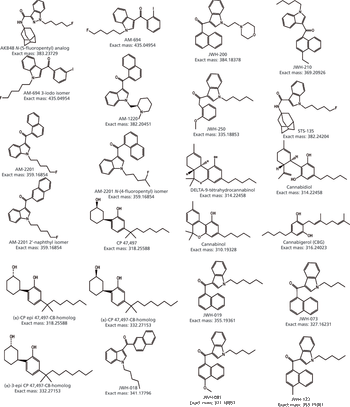
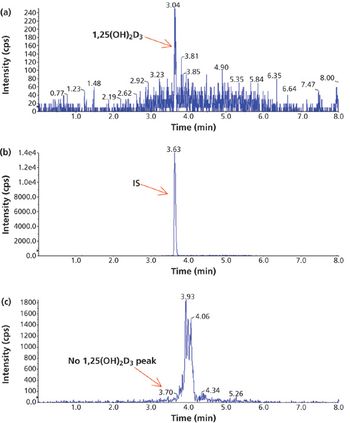
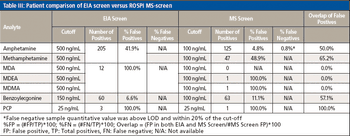
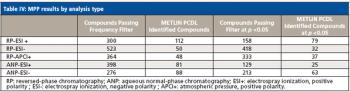
Water samples were obtained from the Tar River and a local water treatment plant in eastern North Carolina in spring 2013 and fall 2015 to monitor the presence of a panel of pharmaceutical and personal care products (PPCPs). Samples were extracted by solid phase extraction (SPE) or liquid-liquid extraction and analyzed for parent PPCPs and their metabolites by liquid chromatography-time of flight mass spectrometry (HPLC-TOFMS) and gas chromatography-mass spectrometry (GC-MS). Both extraction and detection methods were compared by their recoveries and detection limits for each compound. Many parent PPCPs and their metabolites were detected including: carbamazepine, iminostillbene, oxcarbazepine, epiandrosterone, loratadine, β-estradiol, triclosan, and others. Liquid-liquid extraction was found to give overall superior recoveries. Furthermore, HPLC-TOFMS gave lower detection limits than GC-MS. Library searching of additional peaks identified further compounds with biological activity. Additionally, the effectiveness of the treatment plant on the removal of the compounds of interest is discussed.