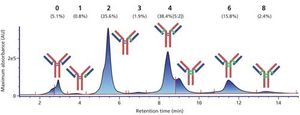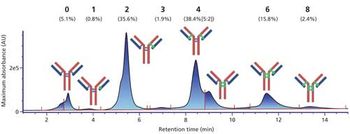
We have developed a range of analytical workflows using mass spectrometry, in a regulated environment, to support pharmaceutical companies in the development and control of their monoclonal antibodies (mAbs) and antibody-drug conjugates (ADCs). High-resolution mass spectrometry is a powerful tool for the analysis of antibodies, but is not readily compatible with a number of chromatographic techniques using high-salt mobile phases. Herein, we present the development and use for marketed mAbs and ADCs of 2D LC–MS via an online desalting step. We demonstrate the importance of such a setup for the determination of drug:antibody ratio (DAR), and the analysis of molecularity, fragmentation, and charge variants (deamidation, oxidation), notably under stress conditions. We discuss the advantages of 2D LC–MS in a regulated environment.