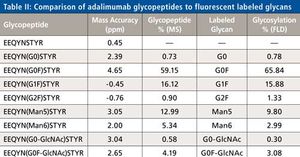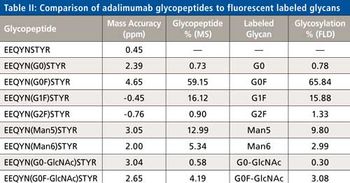
Monoclonal antibodies (mAbs) have been increasingly used as biotherapeutic agents and a number of new mAbs are currently in the drug pipeline. Over the next five years the patent on at least nine major biotherapeutic monoclonal antibodies will expire, opening the door for development and marketing of generic forms known as Biosimilars. In this paper a review of the central role mass spectrometry coupled to liquid chromatography plays in characterizing these antibodies is presented. Contemporary top down and middle-up approaches using mass spectrometry and various novel separation techniques to measure the intact masses of mAbs and their subunits or domains are highlighted. Example data of an innovator mAb, Humira (adalimumab) are presented showing the identities and relative abundances of the isoforms associated with this mAb. Similarly the current state of classical peptide mapping using reversed-phase chromatography and tandem mass spectrometry with scan- dependent acquisition is briefly reviewed. Novel approaches that speed analysis and provide information on post translational modifications, glycosylation, and disulfide mapping are discussed. Example data of stressed and unstressed samples of adalimumab are also presented to demonstrate peptide mapping data and modifications to the antibody. Lastly, the current use of mass spectrometry in glycoprofiling of mAbs is reviewed. Example glycan data for adalimumab generated by a novel labeling scheme and sensitive to detection by both fluorescence and mass spectrometry will be presented.