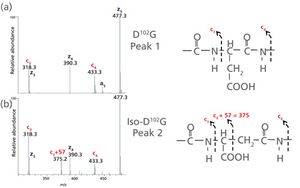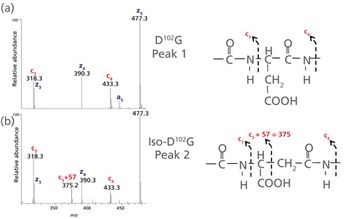
Accurate evaluation of chemical modifications such as asparagine deamidation and aspartic acid isomerization is an essential component of comprehensive characterization of therapeutic monoclonal antibodies (mAbs). When located in the complementarity determining regions (CDRs), these modifications can cause a loss of function, impacting product efficacy and safety, resulting in the designation of the modification as a critical quality attribute. However, artifactual modifications can be introduced by analytical procedures, and distinguishing modifications as either critical quality attributes or method-induced artifacts is an important objective for product development. Conventional peptide mapping coupled with ultrahigh-resolution mass spectrometry offers advanced capabilities for definitive characterization of protein therapeutics. However, experimental conditions such as digestion time and pH can influence the observed level of chemical modifications, usually leading to over-estimation. In this work, a new peptide mapping method was developed specifically for mAb characterization that employs optimal enzyme pH for robustness, but short digestion times and time-course elements to minimize and monitor deamidation/isomerization, respectively, allowing a more accurate assessment of potential CDR sequence liabilities.