The Application of Atomic Spectroscopy Techniques in the Recovery of Critical Raw Materials from Industrial Waste Streams, Part I
This month’s column is a contribution from my daughter Glenna, who recently completed her PhD studies in environmental science from the University of Copenhagen in Denmark. Her article explores the current landscape of global critical raw materials (CRM) trends in research and the applications of atomic spectroscopy (AS), including inductively coupled plasma–mass spectrometry (ICP–MS), inductively coupled plasma–optical emission spectrometry (ICP–OES), and X-ray analytical techniques in their identification of diverse industrial and environmental media, which have been essential in method validation and quantification of CRMs in complex matrices presenting high risks of interference. Some important examples that are presented include rare earth elements (REE) in water leaching purification (WLP) residues that co-occur with radio- active materials, REEs and other metals in acid mine drainage (AMD) environments, REEs in coal combustion (fly ash) residues, arsenic (As) from groundwater treatment sediment, and platinum-group elements (PGE) from sewage sludge. In addition, the article classifies the different techniques in use at each stage of the CRM recovery train, investigate present challenges to each analytical method, and discuss the problem-solving tools used.
- Robert Thomas, Column Editor
This column will be presented in two parts. The first part focuses on identifying the different types of raw materials that will be required, including critical minerals and elements, which have an impact on the green economy, transportation, electronics, and defense technology sectors. The second part of the column (appearing in the June/July issue) will address the analytical challenges encountered by researchers in analyzing these types of samples with atomic spectroscopic techniques, and how they can be overcome.
What Are Critical Raw Materials?
There is a growing demand for the sustainable supply of critical raw materials (CRM) that will support our globe’s transition toward a green and circular economy. The term CRMs (or, as they are sometimes classified, “critical materials,” “critical elements,” and “critical minerals”) refers to a growing list of elements and raw materials that are considered part of a particular economic and strategic interest for key industries and global economies while having a high risk of supply chain disruption (1). Supply chain disruption might occur because of a monopoly of CRM production by a single country, limited global reserves of a CRM, or the rate of consumption exceeding the rate of production. The core industries reliant on CRMs are those related to green energy, transportation, electronics, and defense technology.
The list of CRMs is determined by different governing bodies and often changes. The U.S. Department of Energy most recently compiled a list as of August 2023 with two distinct categories of CRMs—critical materials for energy and critical minerals—while the European Commission published a single list for critical raw materials in March 2023 along with accompanying legislation in The Critical Raw Materials Act (Tables I and II) (1,2).
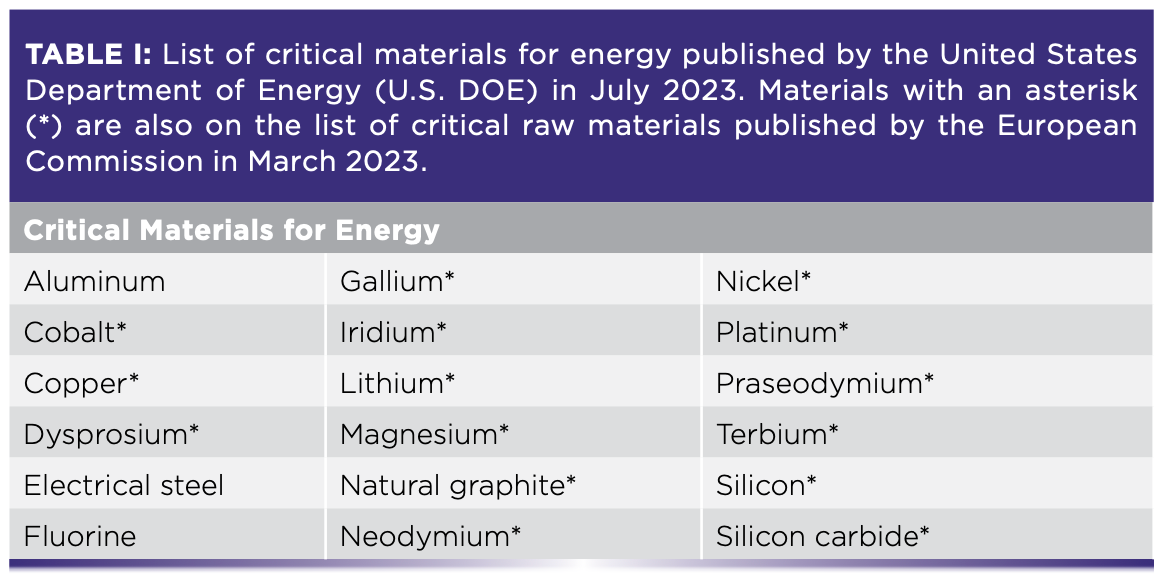
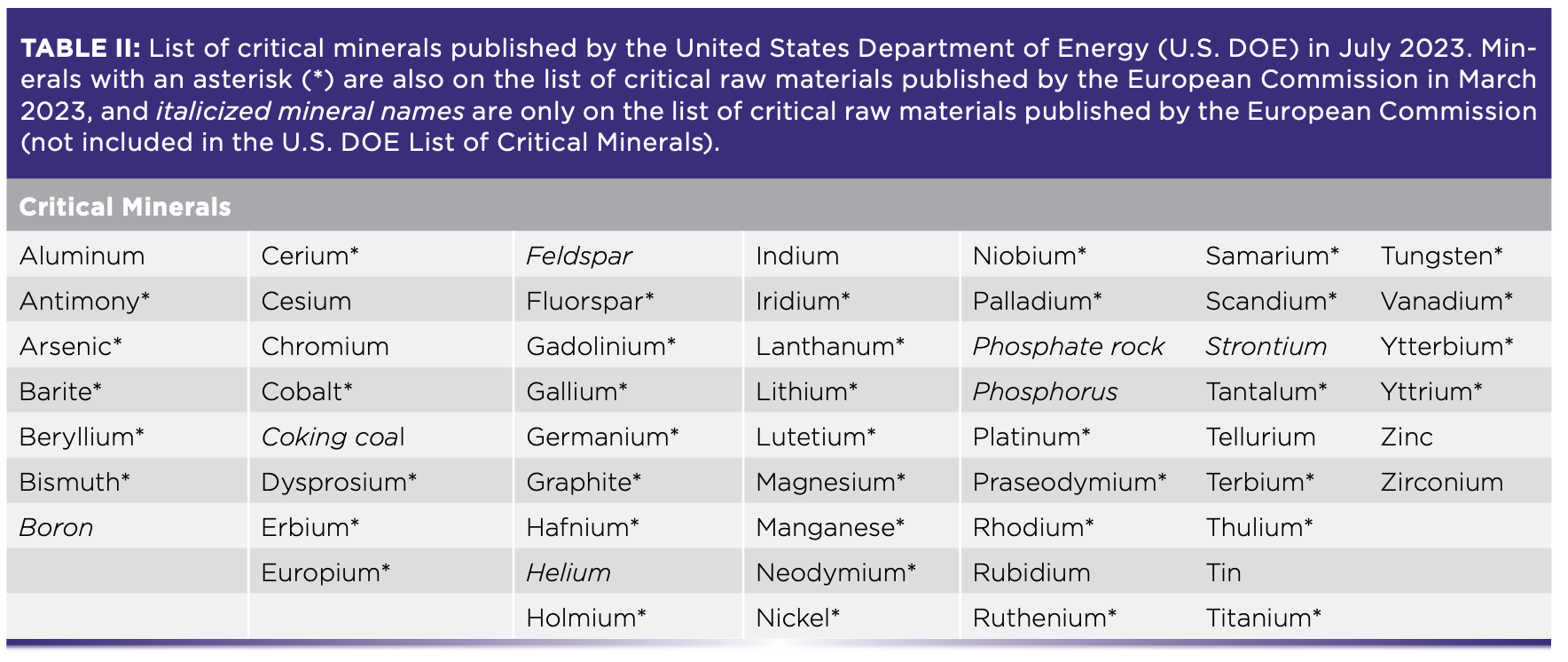
The United Nations has also emphasized the importance of aligning CRM objectives with sustainable development, particularly the Paris Agreement, the international treaty on climate change (3). A task force launched in January 2022, entitled “Working Group on Transforming the Extractive Industries for Sustainable Development,” is devoted to such discussions on supporting the transition towards green and circular economies (4). The current geopolitical and industrial emphasis on CRMs (and, in particular, the sustainable sourcing of CRMs) relies heavily on scientific expertise to enable the transformation of the supported industries.
Research has turned to various industrial waste streams as secondary sources for CRMs. These waste products (such as acidic mine waters, coal combustion ash, water treatment plant residues, mineral and metal processing plant residues, and mine tailings leachate) show a potential for CRM recovery that can complement waste treatment or remediation. Research and development is underway, but for the large part, most technologies are still at laboratory scale and have yet to be commercialized. With certain threats to CRM supply chains, the upscaling of these innovative technologies is critical for establishing renewable resources. This will require cutting-edge analytical techniques at every step along the way to ensure scientific and economic resources are being efficiently invested. Atomic spectroscopy (AS) has been essential in this journey. Whether it is identifying CRMs in waste streams, understanding phase chemistry, testing solubility, or separating CRMs from co-occurring minerals and compounds, AS is one of the most resorted-to suites of analytical techniques in CRM research and recovery.
Applications of Atomic Spectroscopy for the Analysis of Critical Raw Materials
This part of the article focuses on two important CRM groups—rare earth elements (REE) and platinum group elements (PGE)—as well as arsenic (As), which was recently added as a CRM under European legislation (2).
REEs comprise the lanthanide elements on the periodic table with atomic numbers (Z) 57–71, lanthanum (La) through lutetium (Lu), and sometimes includes scandium (Z = 21) or yttrium (Z = 39) because of their similar chemical characteristics and geological occurrences (5). When including yttrium only, the grouping is referred to as “rare earth elements plus yttrium” (REY). REEs may be further divided into categories of “light” and “heavy” based on their ionic radii and atomic mass, although there can be disagreement in literature of the exact delineations. Light rare earth elements (LREEs) typically include lanthanum (La), cerium (Ce), praseodymium (Pr), neodymium (Nd), samarium (Sm), and europium (Eu), whereas heavy rare earth elements (HREEs) include gadolinium (Gd), terbium (Tb), dysprosium (Dy), holmium (Hm), erbium (Er), thulium (Tm), ytterbium (Yb), and lutetium (Lu). Promethium (Pm) occurs in extremely low concentrations in the earth’s crust and is generally neither analyzed nor included in subcategories. Although there are many secondary sources of REEs, current literature focuses largely on acidic mine waters and mine waste material (6) and coal combustion (fly and bottom) ash (7).
The platinum group elements (PGE) comprise ruthenium (Ru) (Z = 44), rhodium (Rh) (Z = 45), palladium (Pd) (Z = 46), osmium (Os) (Z = 76), iridium (Ir) (Z = 77), and platinum (Pt) (Z = 78). They are major components of catalytic converters in cars, and they can be found in concentrated levels along roadsides (in soils and drainage water) (8). Besides primary sourcing from PGE mines, secondary sources include PGE mining wastewater and waste deposits from a number of other metal-ore mines where PGE co-occur in the geological bedrock (9). PGEs are considered critical because of the potential for socio-economic and geopolitical factors to disrupt their supply chain rather than decreasing reserves (8).
Arsenic (As) is a naturally occurring metalloid (Z = 33) used in electronics, pesticides, pharmaceuticals, and as an alloying agent (10). There are many geogenic and anthropogenic sources of As contamination around the world that threaten public health–most notably from As-contaminated groundwater sources (15). Groundwater treatment plants that remove As produce vast quantities of As-rich residues and sludge that require hazardous waste treatment; however, the recovery and recycling of arsenic from groundwater waste residues is a relatively new concept in research and industry (15).
REEs, PGEs, and As are typically found at parts per billion (ppb), micrograms per liter (µg/L), or micrograms per kilogram (µg/kg) concentration levels in the natural environment, depending on units and sample material. However, they are often significantly higher–parts per million (ppm), milligrams per liter (mg/L), or milligrams per kilogram (mg/kg)–in industrial waste products (residues, deposits, tailings, leachate) due to anthropogenic processes of enrichment (11,12). That being said, determination of these elements in both environmental and industrial samples require analytical techniques with a high degree of sensitivity (low detection limits). Industrial waste products have incredibly complex matrices that necessitate sample dilution for instrument operation. This ends up lowering CRM concentrations back to ppb levels in an analyzed solution.
Although a range of techniques have been and can be employed for determination of REEs, PGEs, and As, mass spectrometry (MS), emission spectrometry, and x-ray spectroscopy are the most preferred. In recent years, research has explored such applications towards a variety of industrial waste products with CRM recovery potential. Amidi and colleagues (2020) used X-ray fluorescence (XRF), inductively coupled plasma–mass spectrometry (ICP–MS), and scanning electron microscopy with energy dispersive X-ray spectroscopy (SEM–EDX) to characterize and quantify (88367 ppm) REEs in water leach purification residues from Malaysai containing high levels of iron (32%) and phosphate (20.8%) (12). Pyrgaki and associates used ICP–MS to characterize REE contents in coal fly ash from Greece, achieving lower detection limits of 0.01 and 0.05 ppm (13). Taylor and associates analyzed the REE contents of iron oxide-apatite deposits (IOA) from magnetite mines in the Adirondack Mountains in the United States through wavelength dispersive X-ray fluorescence spectrometry (WDXRF), ICP–MS, and ICP–OES (14). Wang and colleagues studied the molecular scale solid-phase characteristics of As- and Fe-rich groundwater treatment sludge from treatment plants located in Europe, India, and the United States using Fe and As K-edge X-ray absorption spectroscopy (XAS) (15). Using XRF and ICP–OES, Zheng and colleagues analyzed Pt, Pd, and Rh in leaching residues from spent auto-exhaust catalysts from recycling enterprises in China (16). These are just a few recent examples of the research trend towards the characterization and analysis of more chemically complex and diverse industrially sourced samples, which help decision makers distribute the resources needed for developing these technologies at local and global scales.
The second part of this column addresses the analytical challenges encountered by researchers in measuring these elements by atomic spectroscopic techniques and how they can be overcome, and will be published in the June/July issue.
References
(1) Department of Energy. Notice of Final Determination on 2023 DOE Critical Materials List. Document Citation: 88 FR 51792. 08/04/2023. https://www.federalregister.gov/d/2023-16611 (accessed 03/26/2024)
(2) European Commission. Proposal for a Regulation of the European Parliament and of the Council Establishing a Framework for Ensuring a Secure and Sustainable Supply of Critical Raw Materials and Amending Regulations (EU) 168/2013, (EU) 2018/858, 2018/1724 and (EU) 2019/1020 (2023/0079 (COD). Brussels, 03/16/2023.
(3) UNECE. UN Urges Coordinated Action to Align Soaring Critical Raw Materials Extraction with Use with Sustainable Development. 12/06/2023. https://unece.org/climate-change/press/cop28-un-urges-coordinated-action-align-soaring-critical-raw-materials (accessed 03/26/2024).
(4) UNECE. The Working Group on Transforming the Extractive Industries for Sustainable Development. https://unece.org/unece-and-sdgs/working-group-transforming-extractive-industries-sustainable-development (accessed 03/26/2024)
(5) Migaszewski, Z.M.; Gałuszka, A. The Characteristics, Occurrence, and Geochemical Behavior of Rare Earth Elements in the Environment: A Review. Crit. Rev. Environ. Sci. Technol. 2015, 45 (5), 429–471. DOI: 10.1080/10643389.2013.866622
(6) Hermassi, M.; Granados, M.; Valderrama, C.,; Ayora, C. ; Cortina, J.L. Recovery of Rare Earth Elements from Acidic Mine Waters by Integration of a Selective Chelating Ion-Exchanger and a Solvent Impregnated Resin. J. Environ. Chem. Eng. 2021, 9 (5), 105906. DOI: 10.1016/j.jece.2021.105906
(7) Slavković-Beškoski, L.; Ignjatović, L.; Bolognesi, G.; Maksin, D.; Savić, A.; Vladisavljević, G.; Onjia, A. Dispersive Solid–Liquid Microextraction Based on the Poly (HDDA)/Graphene Sorbent Followed by ICP-MS for the Determination of Rare Earth Elements in Coal Fly Ash Leachate. Metals 2022, 12 (5), 791. DOI: 10.1016/j.trac.2019.115708
(8) Komendova, R. Recent Advances in the Preconcentration and Determination of Platinum Group Metals in Environmental and Biological Samples. TrAC, Trends in Anal. Chem. 2020, 122, 115708. DOI: 10.1016/j.trac.2019.115708
(9) Grilli, M.L.; Slobozeanu, A.E.; Larosa, C.; Paneva, D.; Yakoumis, I.; Cherkezova-Zheleva, Z. Platinum Group Metals: Green Recovery from Spent Auto-Catalysts and Reuse in New Catalysts—A Review. Crystals 2023, 13 (4), 550. DOI: 10.3390/cryst13040550
(10) Chen, Q.Y.; Costa, M. Arsenic: A Global Environmental Challenge. Annu. Rev. Pharmacol. Toxicol. 2021, 61, 47–63. DOI: 10.1146/annurev-pharmtox-030220-013418
(11) Wu, G.; Shi, N.; Wang, T.; Cheng, C. M.; Wang, J.,; Tian, C.; Pan, W. P. Enrichment and Occurrence Form of Rare Earth Elements During Coal and Coal Gangue Combustion. Environ. Sci. Pollut. Res. 2022, 29 (29), 44709–44722. DOI: 10.1007/s11356-022-18852-5
(12) Amidi, A.; Razif, S. A .M., Jabit, N. A.; Ariffin, K. S. Characterization of Rare Earth Elements (REE) from Industrial REE Waste Resources. Mater. Today: Proc. 2022, 66, 3140–3143. DOI: 10.1016/j.matpr.2022.07.464
(13) Pyrgaki, K.; Gemeni, V.; Karkalis, C.; Koukouzas, N.,; Koutsovitis, P; Petrounias, P. Geochemical Occurrence of Rare Earth Elements in Mining Waste and Mine Water: A Review. Minerals 2021, 11 (8), 860. DOI: 10.3390/min11080860
(14) Taylor, R. D.; Shah, A. K.; Walsh, G. J.; Taylor, C. D., Geochemistry and Geophysics of Iron Oxide-Apatite Deposits and Associated Waste Piles with Implications for Potential Rare Earth Element Resources from Ore and Historical Mine Waste in the Eastern Adirondack Highlands, New York, USA. Econ. Geo. 2019, 114 (8), 1569–1598. DOI: 10.5382/econgeo.4689
(15) Wang, K.; Holm, P. E.; Trettenes, U. B.; Bandaru, S. R. S.; van Halem, D.; van Genuchten, C. M. Molecular-Scale Characterization of Groundwater Treatment Sludge from Around the World: Implications for Potential Arsenic Recovery. Water Res. 2023, 245, 120561. DOI: 10.1016/j.matpr.2022.07.464
(16) Zheng, H.; Ding, Y.; Wen, Q.; Zhao, S.; He, X.; Zhang, S.; Dong, C. Slag Design and Iron Capture Mechanism for Recovering Low-Grade Pt, Pd, and Rh from Leaching Residue of Spent Auto-Exhaust Catalysts. Sci. Total Environ. 2022, 802, 149830. DOI: 10.1016/j.scitotenv.2021.149830
Glenna Thomas is an environmental research scientist and science illustrator who recently completed her PhD in Environmental Science at the University of Copenhagen in Denmark. Her research focuses on sustainable remediation techniques for soil and water contamination as well as recovery technology for critical materials. She has a background in geology, soil science and pedology and specializes in analytical techniques for the measurement of rare earth elements and heavy metals. Glenna is currently unaffiliated and resides in Christchurch, New Zealand. ●


Newsletter
Get essential updates on the latest spectroscopy technologies, regulatory standards, and best practices—subscribe today to Spectroscopy.
New Study Expands Nickel Autoionization Spectra to Advance Laser Isotope Separation Technologies
July 17th 2025Researchers at China’s National Key Laboratory have identified 170 nickel autoionization states using resonance ionization mass spectrometry, significantly advancing the spectral database critical for laser isotope separation and atomic spectroscopy.
AI-Powered Fusion Model Improves Detection of Microplastics in the Atmosphere
July 17th 2025Researchers from Nanjing University of Information Science & Technology have introduced a breakthrough AI-enhanced multimodal strategy for real-time detection of polyamide microplastics contaminated with heavy metals.
How Analytical Chemists Are Navigating DOGE-Driven Funding Cuts
July 14th 2025DOGE-related federal funding cuts have sharply reduced salaries, lab budgets, and graduate support in academia. Researchers view the politically driven shifts in priorities as part of recurring systemic issues in U.S. science funding during administrative transitions. The impact on Federal laboratories has varied, with some seeing immediate effects and others experiencing more gradual effects. In general, there is rising uncertainty over future appropriations. Sustainable recovery may require structural reforms, leaner administration, and stronger industry-academia collaboration. New commentary underscores similar challenges, noting scaled-back graduate admissions, spending freezes, and a pervasive sense of overwhelming stress among faculty, students, and staff. This article addresses these issues for the analytical chemistry community.
How Do We Improve Elemental Impurity Analysis in Pharmaceutical Quality Control?
May 16th 2025In this final part of our conversation with Harrington and Seibert, they discuss the main challenges that they encountered in their study and how we can improve elemental impurity analysis in pharmaceutical quality control.