
The authors discuss the use of electron-capture dissociation coupled with a linear ion trap time-of-flight mass spectrometer to investigate the structure of human transferrin.
Masaki Watanabe is with Hitachi High-Technologies, Hitachinaka, Ibaraki, Japan.

The authors discuss the use of electron-capture dissociation coupled with a linear ion trap time-of-flight mass spectrometer to investigate the structure of human transferrin.

The authors introduce a compact ECD device coupled to a linear ion trap time-of-flight instrument, and use it to analyze protein phosphorylation in both offline and online modes.
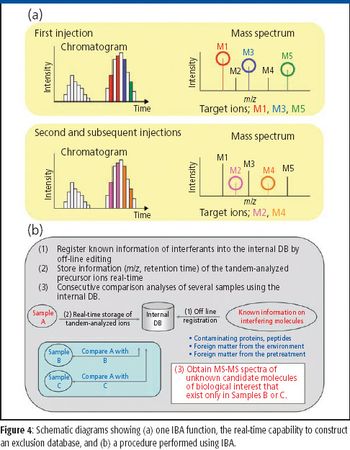
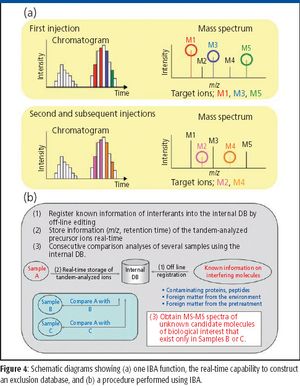
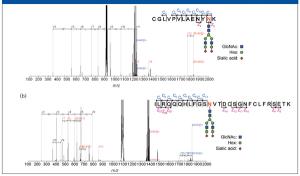
Mass spectrometers are effective for identifying and quantifying unknown molecules, such as disease-related proteins and small molecules in pharmaceutical research and medical diagnosis. In addition, mass spectrometry (MS) can be particularly powerful when analyzing molecules with complex structures, such as posttranslationally modified proteins. Among various MS approaches, high-resolution multistep tandem MS (MS-MS) is an emerging methodology for accurate identification of complex molecules. In this article, we describe a new approach for mass analysis with enhanced quantitative capability combined with high-resolution multistep MS-MS, where the dynamic range of quantitation covers four orders of magnitude.
Published: April 1st 2007 | Updated:

Published: July 1st 2008 | Updated:
Published: November 1st 2008 | Updated: