Article
Spectroscopy
Spectroscopy
Organic Secondary Ion Mass Spectrometry
Ionization strategies and themes first developed in organic secondary ion mass spectrometry underlie many modern MS methods. Here's how it works.

Secondary ion mass spectrometry (SIMS) is a mass spectrometry (MS)-based method used in surface analysis, and organic SIMS is used to probe organic, polymeric, and biomolecular structures supported on surfaces. Ionization strategies and themes first developed in organic SIMS underlie many modern MS methods of analysis. Here, we explain how it works.
Reading through a scientific publication, one might encounter the sentence: "The sample was analyzed by mass spectrometry (MS)." Long-time readers of this column might immediately seek additional details. What ionization method was used? Positive or negative ions? Low- or high-mass resolution measurement? Was MS-MS involved? Was the analysis targeted at single-compound identification or mixture analysis? More broadly, what was the reason for the analysis? What other analytical data were measured? Where did the sample come from, and what sort of sample was it? What was the conclusion supported by the data? In our minds, we may create this vision of a "sample," and a common assumption for the analysis is that the sample (solid, liquid, or gas) is captured in a container and transported to the MS instrument. But the sample can also be a surface, described simply in two spatial dimensions (x and y). We can create ions just from that surface, and separate those ions by mass to measure a mass spectrum providing information about what atoms and molecules reside at the surface, and how they might be connected. Secondary ion mass spectrometry (SIMS) is an MS-based method used in surface analysis, and organic SIMS is used to probe organic, polymeric, and biomolecular structures supported on surfaces. Ionization strategies and themes first developed in organic SIMS underlie many modern MS methods of analysis.
Imagine, if you will (1), a perfectly flat surface. What may appear to be perfectly flat on a laboratory scale becomes progressively less so as the scale of examination decreases. Ultimately, you must conclude that perfectly flat surfaces are only an abstraction. A clean surface consisting of an array of elemental atoms still has horizontal and vertical structure at the atomic scale. Crystal imperfections and dislocations provide structure. For slightly more complex surfaces, different crystal forms, atomic substitutions, and adsorbed atoms and molecules all add to the topology. It is understood that any surface not held under a very good vacuum is covered with adsorbed gases, often held there by noncovalent bonds, but occasionally held by stronger interactions. Some fraction of these gases may remain adsorbed on the surfaces even at higher temperatures and lower pressures. Sputter cleaning (2,3) removes these adsorbents by bombardment-induced erosion of the outermost layers of a surface, but may itself rearrange the surface constituents. The surface may subsequently be reconstructed (annealed). Understanding and controlling these transformations and creating a clean reactive surface underlie surface treatments such as plating, layering, passivation, and catalysis. The atomic environment in these processes may encompass a few tens of atoms to perhaps a few hundred atoms, with patterns repeated across a larger (x,y) dimension of the surface. SIMS is one of the analytical methods used to analyze these surfaces, and it provides information about atomic and smaller inorganic species and their (x,y) distribution, as well as the depth profiles of these species.
The Fundamentals of SIMS
Although SIMS is usually described as an analytical method, as described above, it also is a distinct ionization process. The types of mass analyzers and detectors used in SIMS instruments parallel those used in other forms of MS, and so they will not be discussed further in this installment. We will focus on the fundamental process in SIMS ionization, as shown in Figure 1. The surface (either serving itself as the sample or as a support with other material adsorbed or deposited on it) is represented as the horizontal line at the bottom of the figure. The surface is held under vacuum. A primary beam of particles (ions for SIMS, or atoms for fast atom bombardment [FAB]) is created in a source, collimated, and aimed at the surface, usually impacting at a geometrical angle of about 45°. The kinetic energy of the primary particle is usually several kiloelectronvolts (or equivalent). The flux of incoming particles in the primary beam, its spatial profile (footprint), its mass, and even its chemical reactivity are experimental parameters that may be varied. In most applications, the incident particle beam is chosen to be inert, and for experimental ease, a beam created from argon ions or atoms is often used. In a simple approximation, the energy deposited into the sample and surface layers through primary particle impact can be modeled in a "billiard-ball" model. The energy propagates via multiple collisions through the surface and into the bulk. The bulk is defined as extending a few tens of angstroms below the surface. Accordingly, even a "surface" becomes a volume, although one in which the surface dimensions may be much larger than the depth dimensions. The deposited energy propagates through this microvolume and changes its character. For instance, kinetic energy can become thermal energy or cause electronic excitation. In the collision model, some fraction of the incident energy returns to the surface and through direct collision releases surface atoms and adsorbed gases into the vacuum. These released particles are "secondary," hence the name secondary ion mass spectrometry. How do the secondary particles become ionized? In fact, only a fraction of the particles released from the surface are ions, and a lot of research has resolved several different forms of desorption and ionization, including a linear cascade model and multibody collision models (4,5). Desorbed neutral species diffuse through the vacuum. Extraction optics in the SIMS instrument are designed to gather secondary ions from the surface, concordant with the spatial resolution required, and direct them into the mass analyzer.
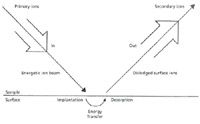
Figure 1: Generalized schematic for SIMS, showing the primary particle beam impacting the surface (horizontal line) and sputtered secondary ions extracted into the mass spectrometer. Secondary neutral species diffuse into the vacuum.
If these particles are ionized directly at the site of primary ion impact, then the spatial origin of these ions is known and the surface can be imaged with a resolution concordant with the primary beam footprint. This is the classical SIMS analysis of surfaces, and larger instruments with special optics provide imaging SIMS capabilities (6).
The Origin of Organic SIMS
Beginning in earnest in the early 1980s, organic SIMS (sometimes called molecular SIMS to differentiate it from inorganic SIMS of mostly atomic species) was developed as a means to generate mass spectra of nonvolatile organic compounds adsorbed on, or supported by, surfaces. Ionization methods such as electron ionization or chemical ionization could be used for organic compounds that could be evaporated into the gas phase, but the nonvolatile compounds, even of lower mass, were problematic for conventional MS ionization methods. SIMS and organic compound analysis had intersected before. Honig first purposefully introduced organic compounds into a SIMS instrument in 1962 (7) and foresaw the potential of SIMS to provide information about the identity of such compounds and how they were arrayed on a surface. However, for many years afterwards, most practitioners thought of organic compounds only as contaminants. The adsorbates were residual gases, and organic compounds derived from pump oils and lubricants were of no interest in and of themselves. Sputter cleaning (described above) was used to prepare a clean surface for analysis. In fact, under the conditions of primary beam flux usually used in inorganic SIMS, organic molecules at the surface were excessively fragmented, providing only lower-mass carbon-containing ions in the mass spectrum rather than an ion corresponding to the molecular mass M. These ions provided little information about molecular structure and could become isobaric interferences for the atomic ions that were desired.
In the early 1970s, Benninghoven (8) developed what is called the static SIMS technique. Even though higher primary ion fluxes could be used to sputter-clean the surface, and then to depth profile through a surface layer-by-layer (termed dynamic SIMS), lower primary ion fluxes created a sampling condition in which it was unlikely that the recorded mass spectrum would contain products of beam-induced surface reactions. The surface could be considered static and unchanged by the primary beam impact; hence the name static SIMS. The flux of secondary ions was reduced, but even at lower absolute signal levels for secondary ions, it soon became clear that the method had great potential for analysis of nonvolatile organic compounds supported on surfaces. How might such a sample be prepared? Gaseous organic compounds could be condensed onto support surfaces. But liquid and solid samples presented a different sample preparation challenge. Thin layers of organic compounds could be deposited on surfaces with specialized techniques (such as the Langmuir–Blodgett methods), but what was needed for organic SIMS was a method of sample surface preparation that was as convenient as those used in other forms of MS and could be used for organic compounds that could not be analyzed by methods such as electron ionization or chemical ionization MS.
In much of the early work, the sample was burnished in thin layers onto a metal support surface so that the primary beam sampled both metal and organic compound. Although the organic compound could be desorbed as a neutral secondary species, the sputtered metal ions could react with the neutral molecule to create the cationized organic species. As an example, in much of the early work, silver was used as the support metal. Sputtering would produce both Ag+ and desorbed neutral organic molecules. The process of cationization would produce (M+Ag)+ ions analogous to the protonated molecule (M+H)+ produced in positive-ion chemical ionization. The goalpost isotopes of silver (107 Ag and 109 Ag, in near equal abundances) are clearly seen in the recorded mass spectrum and provide a double-check on the value of the molecular mass M. Cationization of organic molecules (9,10) with metals produces ions of novel structures; producing them can be as simple as placing a thin layer of the organic compound onto the metal support and sputtering with the primary particle beam.
It soon became clear that the sputtering of a surface by a primary particle beam created a chemically reactive environment right above the surface. This region, called the selvedge, is where sputtered neutral species and secondary ions interact with each other (similar to what is seen in a chemical ionization source). With multiple collisions just above the surface, and energy to drive reactions, the final charge carrier leaving the selvedge and extracted into the mass analyzer should be the most stable ionic species. Of course, the effective pressure drops rapidly with distance from the surface and collisions cease. Unimolecular dissociations of ions may subsequently occur just as in other ionization methods. An intriguing feature of organic SIMS is the fact that potential reactants can be added to the mix simply by adding them to the area bombarded by the beam. As the reactants became more complex, so did the range of possible reactions, and the range of secondary ions observed.
Ionization strategies first developed in organic SIMS offered a preview of later developments in other MS methods for the analysis of nonvolatile organic compounds. Realizing that the selvedge was often the actual site of ionization, additives to the surface mixture were designed to create a favorable ionization mixture. Accordingly, matrix-assisted SIMS experiments were described starting in 1981 (11,12). The most common matrix was ammonium chloride, chosen for its ability to moderate the energy of the primary beam and create a favorable environment in the selvedge for protonation of the neutral sample molecule to form (M+H)+ ions from neutral sample molecules M. The principle of matrix assistance was later used to develop matrix-assisted laser desorption–ionization (MALDI) MS. The ease in which preformed ions could be observed in the organic SIMS mass spectra also was soon apparent (13), an observation that was generalized across almost all of the desorption ionization methods concurrently developed for the analysis of nonvolatile organic compounds. This principle is being expanded in current research with increasingly sophisticated models of ion formation in MALDI (14).
Organic SIMS and Fast Atom Bombardment
Organic SIMS overlapped for a time with a MS ionization method known as fast atom bombardment (FAB). Substitution of a primary neutral beam for a primary ion beam (a simple change in the nature of the primary particle; see Figure 1) provided certain instrumental advantages (and disadvantages, as it turned out), but this substitution did not change the fundamental ionization processes. The useful characteristic of FAB was its use of a liquid surface. The organic sample was dissolved in a semivolatile liquid (usually glycerol, although a few other liquids were commonly used), and this solution was then placed on the support, and the liquid film was bombarded by the primary particle beam. What distinguishes a solid from a liquid support? Simply stated, the difference is the strength of intermolecular attractions. Billiard-ball collisions can be modeled in both forms of matter, but in the liquid matrix, the cascade will spread further and persist longer. Additionally, liquids (at least those successfully used as matrices in FAB) have a significant vapor pressure relative to the vacuum in the rest of the instrument. Evaporation from the surface is constant, presenting a constantly renewed surface (achieving the same goals as the static SIMS experiment). The impact of the primary particle beam also creates a spray of microdroplets from the liquid surface (this can be seen visually with a microscope), with enhanced evaporation of liquid matrix from those droplets. Some of those droplets could be charged as they left the surface, or they could acquire a charge as they traversed the selvedge. The final steps of ionization for sample molecules dissolved or dispersed in the matrix occurred in those droplets, and an underlying similarity to processes of electrospray ionization is not unexpected. Of course, the ionization processes in the droplets is not directly connected to the charge state of the primary particle, so "liquid SIMS" and FAB were equally capable analytical methods.
SIMS and Beyond
Figure 2 summarizes ionization processes observed (many of them simultaneously) in organic SIMS, FAB, and by extension MALDI and electrospray ionization. The abbreviation scheme is shown in the lower right part of the figure. Although the surface is shown as curved, the thickness of any adsorbed liquid film is unspecified, or there may be no liquid at all. The top process shown is the direct desorption of a cation from the solution into the gas phase; anions are similarly sampled. No ionization is necessary; this is simply a transport process. Derivatization strategies originally developed for organic SIMS to create such preformed ions from organic compounds have migrated with equal usefulness to MALDI and electrospray ionization. Protons, the simplest default carriers of positive charge, are easily formed on surfaces or in liquid solutions, and their reaction with neutral sample molecules M form (M+H)+ ions. The importance of transport processes in liquid SIMS and FAB was revealed when it was found that surface-active species (surf) in a liquid matrix provided early signals, with less surface-active species appearing later in the time course of mass spectra. The analogy to depth profiling through a solid layered surface should be clear. Analogies to electrochemical reactions are seen in the charge separation observed for donor–acceptor complexes, with the positive and negative ions appearing in the respective mass spectra. Charge-transfer derivatization is a demonstrated method to enhance the ionization efficiency in organic SIMS. Cations and anions may cluster together, and these may include intriguing mixtures of inorganic and organic species. With one excess cation, the charge on the cluster is positive, and with one extra anion, the charge is negative. If S represents the solvent matrix, then charged species in the solution may associate with several neutral solvent molecules, and the surviving species sputtered from the surface may retain an association with S (usually only a small number of S), and the solvated ions are observed in the mass spectrum. Finally, the last-listed reaction is analogous to the process of cationization, in which a neutral sample molecule M is ionized by the cation derived from a salt. When the salt is silver chloride, for example, the metal cation carries the positive charge to the molecule, forming (M+Ag)+.
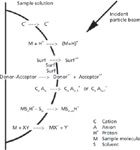
Figure 2: Compilation of ionization processes observed in organic SIMS, with exemplars for positive ions.
Finally, the imaging of surfaces has been a part of SIMS analysis almost since its inception. Movement of the primary beam across the surface to reveal changes in surface structure can yield different mass spectra as a function of location in a simple "image." Rastering the primary ion beam yields an image if the secondary ion optics preserves the spatial information. Such capabilities are optimized in modern ion microprobe instruments. Early on, these capabilities were focused on lower mass atomic and polyatomic species. As our understanding and control of sample ionization and transport processes grew more sophisticated, the imaging capabilities of organic SIMS were accessed (15). Substitution of an incident laser beam for the primary particle beam changes the instrument, but not necessarily the ionization and imaging processes. Accordingly, imaging MALDI (16) has undergone a sustained parallel development.
Kenneth L. Busch spent many late nights working with a Riber SIMS instrument, designed for classical surface analysis, but used as a robust tool to help develop applications of organic SIMS. Experimental success was determined by the nature of the chemistry at the sputtered surface, and not the "approved uses" of the surrounding instrument. As always, the ions know what they are doing, and it is up to us to figure it out. Imagine, if you will, that it should not take us so long to do so. This column is the sole responsibility of the author, who can be reached at WyvernAssoc@yahoo.com

Ken Busch
References
(1) This column prompts the reader to look beyond the SIMS surface, just as the classic show "Twilight Zone" encouraged viewers to take a closer look at the issues of its time. See: http://www.tv.pop-cult.com/twilight-zone.html.
(2) H.E. Farnsworth, R.E. Schlier, T.H. George, and R.M. Burger, J. Appl. Phys. 26, 252–254 (1955).
(3) E. Taglauer, Appl. Phys. A: Mater.Sci. and Proces. 51(3), 238–251 (1990).
(4) P. Sigmund, Nucl. Instr. Meth. Phys. Res. B 27, 1–20 (1987).
(5) Sputtering by Particle Bombardment, R. Behrisch, Ed. (Springer, Berlin, 1981).
(6) A. Benninghoven, F.G. Rüdenauer, and H.W. Werner, Secondary Ion Mass Spectrometry: Basic Concepts, Instrumental Aspects, Applications, and Trends (Wiley, New York, 1987).
(7) R.E. Honig, Adv. Mass Spectrom. 2, 25 (1962).
(8) A. Benninghoven, Surf. Sci. 35, 427–457 (1973).
(9) A. Benninghoven and W. Sichtermann, Org. Mass Spectrom. 12, 595–597 (1977).
(10) H. Grade, N. Winograd, and R.G. Cooks, Amer. Chem. Soc. 99, 7725–7726 (1977).
(11) L.K. Liu, K.L. Busch, and R.G. Cooks, Anal. Chem. 53, 109–113 (1981).
(12) B.H. Hsu, Y.-X. Xie, K.L. Busch, and R.G. Cooks, Int. J. Mass Spectrom. Ion Phys. 51, 225–233 (1983).
(13) K.L. Busch, S.E. Unger, A. Vincze, R.G. Cooks, and T. Keough, J. Amer. Chem. Soc. 104(6), 1507–1511 (1982).
(14) J.H. Moon, Y.S. Shin, Y.J. Bae, and M.S. Kim, J. Amer. Soc. Mass Spectrom. 23, 162–170 (2012).
(15) H. Nygren and P. Malmberg, Trends in Biotechnol. 25(11), 499–504 (2007).
(16) A compilation of information and applications is at: http://www.maldi-msi.org/

Newsletter
Get essential updates on the latest spectroscopy technologies, regulatory standards, and best practices—subscribe today to Spectroscopy.



