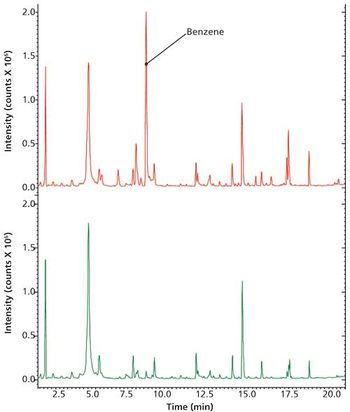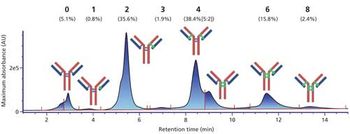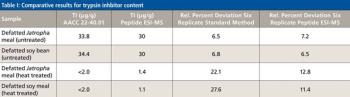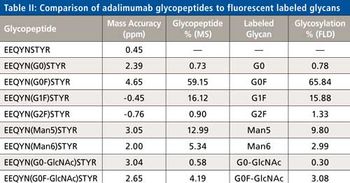
Special Issues
A newly discovered method is described for generating gas-phase ions from volatile and nonvolatile compounds. The method, matrix-assisted ionization (MAI), is both simple and sensitive, requiring only the vacuum inherent with all mass spectrometers and a suitable matrix, eliminating the need for lasers, electric fields, nebulizing gas, and even heaters to generate gas-phase ions. MAI is applicable for the direct analysis of drugs from biological fluids and tissue without prior purification. By placing matrix only on a specific surface area of interest and exposure to the vacuum of the mass spectrometer, ions are observed from compounds within the targeted surface area of tissue exposed to the matrix solution, thus allowing rapid and simple interrogation of “features of interest.” The limit of detection for drug standards is low attomoles and clean full mass range mass spectra are obtained from low femtomoles of the drug.