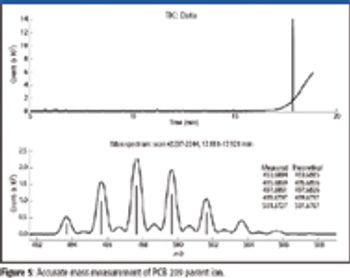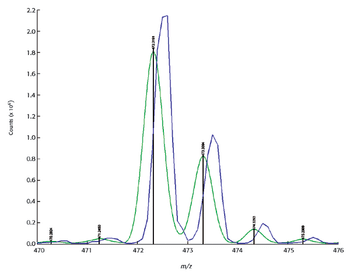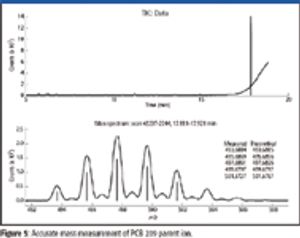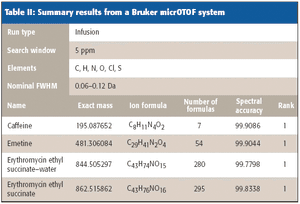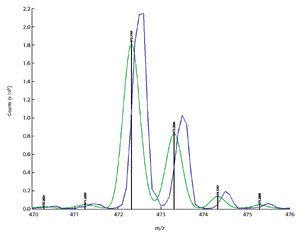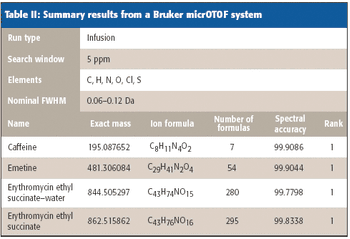
Mass spectrometry has become a fundamental tool for compound identification or confirmation by virtue of its ability to obtain elemental composition determination (formula identification) by accurate mass measurements. The speed, sensitivity, and ease of interfacing the technique with gas chromatography and liquid chromatography make it the technique of choice for many applications. However, accurate mass measurements must be made with care, and sometimes they can require careful calibration procedures and validation methods. In addition to accurate mass measurements, the isotope abundance distribution also provides information unique to a given chemical formula. However, the mass spectral accuracy required for accurate isotope modeling has not been easy to obtain previously. More recent approaches (1–3) that calibrate the spectral line-shape show promise in obtaining the necessary level of spectral accuracy but still require careful calibration methods with the use of known standards. This article..