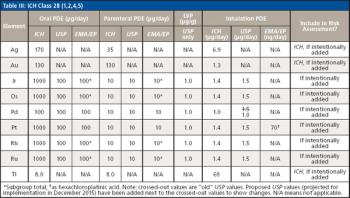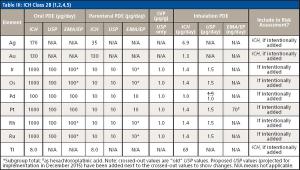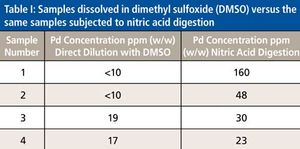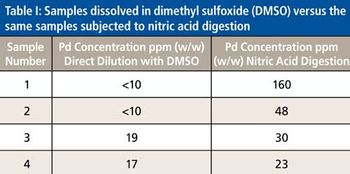
The use of atomic spectroscopy techniques and sample preparation procedures is something that is not as routine in the pharmaceutical industry as are chromatography-based techniques and sample preparation procedures. With new requirements being implemented regarding elemental impurities by the United States Pharmacopoeia (USP) and International Conference on Harmonization (ICH), analysts in the pharmaceutical industry are, in many cases, working to determine how best to analyze their samples. Sample preparation techniques that can be used for pharmaceutical samples are the same ones that have been used by other industries for many years. This paper will provide a brief overview of potential techniques.