
We continue our explanation of the new PIC/S data guidance.
R.D. McDowall is the director of R.D. McDowall Limited and the editor of the “Questions of Quality” column for LCGC Europe, Spectroscopy’s sister magazine.

We continue our explanation of the new PIC/S data guidance.
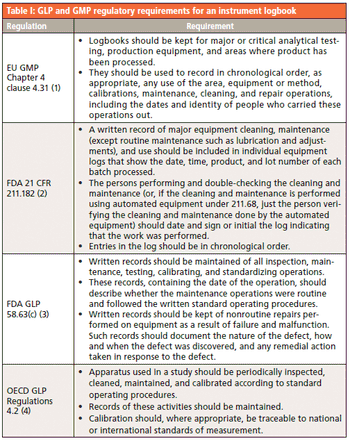
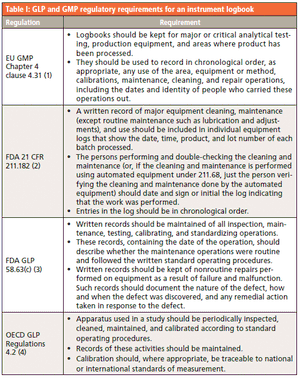
Good practice (GxP) regulations require an instrument maintenance and use log. Have spectroscopy laboratories and software suppliers emerged from the Middle Ages yet?

The new version of United States Pharmacopeia general chapter “Analytical Instrument Qualification” became effective August 1, 2017. What does this mean for you?

Data integrity requires a multilayered approach that runs throughout a regulated organization. Here we discuss the laboratory level.

In April 2016, the FDA issued a draft guidance for industry on data integrity and compliance with cGMP. What does this mean for the laboratory?

Data integrity is currently the hottest topic in regulated laboratories. Understanding what constitutes data integrity and the interactions between the layers is the challenge to ensure that data are accurate, correct and complete. Are you up to the challenge?

Published: December 1st 2021 | Updated:
Published: April 1st 2016 | Updated:

Published: September 1st 2016 | Updated:

Published: April 1st 2017 | Updated:

Published: September 1st 2017 | Updated:
Published: December 1st 2017 | Updated: