- Spectroscopy-09-01-2018
- Volume 33
- Issue 9
The C=O Bond, Part VII: Aromatic Esters, Organic Carbonates, and More of the Rule of Three
Aromatic esters follow the ester Rule of Three, but each of these three peak positions is different for saturated and aromatic esters, which makes them easy to distinguish. Organic carbonates are structurally similar to esters and follow their own Rule of Three.
In this seventh installment of our study of the infrared spectroscopy of the carbonyl group, we look at aromatic esters and carbonates. We see that aromatic esters follow the ester Rule of Three, but that each of these three peak positions is different for saturated and aromatic esters, which makes them easy to distinguish. Organic carbonates are structurally similar to esters and follow their own Rule of Three.
In our last installment (1), I introduced you to the ester functional group. As a review, the molecular structure and the naming of the atoms in an ester group are shown in Figure 1.
Figure 1: The molecular structure of the ester functional group.
Recall that there are two types of esters, depending on the identity of the alpha carbon. If the alpha carbon is saturated, the ester is saturated. If the alpha carbon is aromatic, the ester is aromatic. In the last column, I only had room for a discussion of saturated esters. In this installment, we will finish our discussion of esters by covering aromatic esters.
Organic carbonates as a functional group are similar to esters, and so it is appropriate to group them with aromatic esters. The chemical term carbonate is a little confusing. There exist inorganic carbonates that contain the CO32- ion. Although this functional group contains a carbon atom, it acts inorganic, since it forms ionic bonds and makes up rocks such as limestone, rather than typical organic compounds. Organic carbonates also contain a carbon atom with three oxygens bonded to it, but the bonding is covalent, and the functional group is considered organic. Therefore, it is always important to use the term organic carbonates when discussing the covalent version of this moiety.
Structurally, organic carbonates look like two esters squished together, as is shown in Figure 2.
Figure 2: The molecular framework of an organic carbonate.
As you can see in Figure 2, carbonates are symmetrical, and each half can be thought of as an ester, with each half having a carbonyl carbon, an ester oxygen, and an alpha carbon. Note that the carbonyl carbon has not one oxygen attached to it, as in an ester, but two. This means there are two ester oxygens and two alpha carbons. There are three types of carbonates: saturated, where both alpha carbons are saturated, aromatic, where both alpha carbons are aromatic, and mixed, where one alpha carbon is saturated and one is aromatic. Given the structural similarity between esters and organic carbonates, we might expect them to follow the Rule of Three, and as we will see they do. As we will also see, we can distinguish between the three types of carbonates based on their infrared spectra, and, in general, distinguish between esters and carbonates based on their spectra.
The Infrared Spectroscopy of Aromatic Esters
In the last column (1), we saw that esters have a memorable pattern of three intense peaks at ~1700, ~1200, and ~1100 from the C=O and two C-O stretches, and hence follow what I call the Rule of Three (2). We also saw that saturated esters follow the Rule of Three. Aromatic esters also follow the Rule of Three, but all three peaks for aromatic esters fall in different wavenumber ranges than the peaks of saturated esters do (Figure 3).
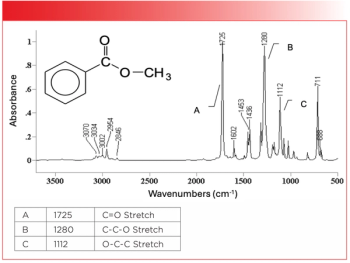
Figure 3: The infrared spectrum of methyl benzoate, an aromatic ester.
The Rule of Three peaks in Figure 3 are labeled A, B, and C, and are easy to spot, sticking up like three long fingers in the middle of the spectrum. The peak labeled A at 1725 is the unmistakable C=O stretch, and is almost always the most intense peak in the spectrum. In general, for aromatic esters this peak falls between 1730 and 1715, and is lower than that of saturated esters because of conjugation (1). Peak B at 1280 is the C-C-O stretch, which is normally found from 1310 to 1250. Lastly, peak C is the O-C-C stretch, which ranges from 1130 to 1100. These three peaks follow the intensity pattern we have seen before for esters, where the first two Rule of Three peaks are more intense than the third. Table I lists the Rule of Three peak positions for aromatic esters, and compares them to those for saturated esters.
Note in Table I that, for each of the three vibrations listed, the ranges for saturated and aromatic esters are different. Each ester type has a unique set of Rule of Three peaks, and any of the peaks can be used to distinguish saturated and aromatic esters from each other.
Quiz Section: Answer to the July Quiz and a New Interpretation Challenge
The Infrared Spectra of Organic Carbonates
As discussed above, organic carbonates come in saturated, aromatic, and mixed forms. The infrared spectrum of an aromatic carbonate, the common polymer Lexan (2,2-bis[4-hydroxyphenyl]propane polycarbonate), is shown in Figure 4.
Figure 4: The infrared spectrum of the aromatic carbonate Lexan.
Lexan is an aromatic carbonate because the two alpha carbons are part of benzene rings. The peak at 1777 is undoubtedly a C=O stretch based on its strength and position. The carbonyl stretch of aromatic carbonates in general falls from 1820 to 1775. This region is higher than that of any ester, and makes it easy to distinguish between aromatic esters and carbonates. The C=O stretch of mixed carbonates falls from 1790 to 1760, and for saturated carbonates this peak is seen at 1740 ± 10.
Esters have a C-C-O stretch that falls around 1200 (1). Carbonates have instead have an O-C-O bond, and the asymmetric stretch of this bond is seen at 1230 in Figure 4. For mixed carbonates, it falls from 1250 to 1210, and, for saturated carbonates, from 1280 to 1240. Lastly, carbonates contain an O-C-C moiety whose asymmetric stretch can be seen at 1015 in Figure 4. This peaks falls between 1060 and 1000 for all types of carbonates.
To review, then, carbonates follow the Rule of Three like esters with three intense peaks at ~1700, 1200, and 1000. These peaks are from the C=O, O-C-O, and O-C-C stretches. The first two are sensitive to the type of carbonate; the O-C-C stretch is not. A summary of the group wavenumbers for organic carbonates is shown in Table II. Note that the saturated carbonate C=O stretch falls in the same range as that of saturated esters (Table I). However, saturated carbonates have their O-C-O peak from 1280 to 1240, whereas for saturated esters, the C-C-O peak is lower, from 1210 to 1160. It is the relative position of these two peaks that allows one to distinguish saturated esters and carbonates from each other. The high-wavenumber C=O stretches of mixed and aromatic carbonates mean they will never be confused with esters.
Conclusions
Like saturated esters, aromatic esters follow the Rule of Three with intense peaks at ~1700, 1200, and 1100 from C=O, C-C-O, and O-C-C stretches. However, as shown in Table I, saturated and aromatic esters have unique Rule of Three peaks, and any of these peaks can be used to distinguish these two types of esters from each other.
Carbonates are similar structurally to esters and also follow their own Rule of Three with peaks from C=O, O-C-O, and O-C-C stretches. Carbonates can be distinguished from esters and each other using the peaks listed in Table II.
References
(1) B.C. Smith, Spectroscopy 33(7), 20–23 (2018).
(2) B.C. Smith, Infrared Spectral Interpretation: A Systematic Approach (CRC Press, Boca Raton, Florida, 1999).
Brian C. Smith, PhD, has more than three decades of experience as an infrared spectroscopist. He has published numerous peer reviewed papers and has written three books on the subject: Fundamentals of FTIR and Infrared Spectral Interpretation, both published by CRC Press, and Quantitative Spectroscopy: Theory and Practice published by Elsevier. As a spectroscopy trainer, he has helped thousands of people around the world improve their infrared analyses. He earned his PhD in physical chemistry from Dartmouth College. He can be reached at:
Articles in this issue
over 7 years ago
A Flexible Analytical Data Life Cycle?over 7 years ago
Infrared Spectroscopy: New Frontiers Both Near and Farover 7 years ago
Spectroscopy September 2018 Regular Issue PDFNewsletter
Get essential updates on the latest spectroscopy technologies, regulatory standards, and best practices—subscribe today to Spectroscopy.