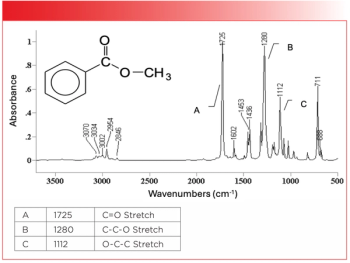
In this part of our ongoing review of the infrared spectra of carbonyl-containing functional groups, we will study the spectra of esters and carbonates. Esters are ubiquitous in our food and medicines, and polymeric carbonates form an important part of the materials around us. As always, concepts will be illustrated with reference spectra.