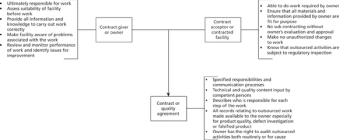
Data governance requires a multi-layered approach that runs throughout a regulated organization from top to bottom. Although data governance features in the majority of GxP data integrity guidance documents, the approach to the topic should be business-driven rather than regulatory-driven.