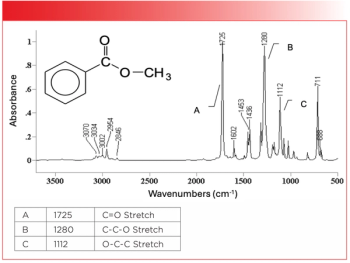
Chad Atkins Wins Young Chemist Award
Chad Atkins has been named the winner of the 2015 Young Chemist Award. Competition for the $10,000 award, from Metrohm USA, is open to all graduate, post-graduate, and doctorate students residing and studying in the United States and Canada, who are performing novel research in the fields of titration, ion chromatography, spectroscopy, and electrochemistry.
Atkins is completing his PhD at the University of British Columbia (Vancouver, BC) where he works under the supervision of Robin Turner and Michael Blades. His research uses Raman spectroscopy to assess the egradation of stored red blood cells to measure age-related changes in stored cell breakdown. By measuring amples through the storage bag, the possibility of contamination is reduced. Confirming the viability of stored red blood cells prior to transfusion leads to a more successful outcome.
This is the third year Metrohm USA and Metrohm Canada have awarded the Young Chemist Award.
“Metrohm has a history of giving back to the scientific community,” said Edward Colihan, president and CEO of Metrohm USA. “This year we saw a record number of applications for this award, demonstrating ingenuity and a passion for solving very practical problems. We are proud to support the next generation of scientists.”
Newsletter
Get essential updates on the latest spectroscopy technologies, regulatory standards, and best practices—subscribe today to Spectroscopy.