Articles by David Gregson
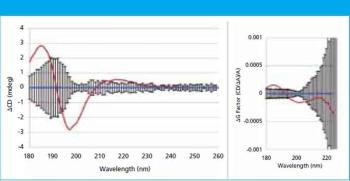
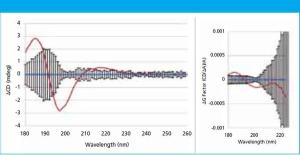
In this study, far-UV CD spectra of eight different mammalian serum albumins were measured repeatedly using automated CD spectroscopy. Two independent methods of normalizing the CD data were used to eliminate the need for accurate knowledge of protein concentration or extinction coefficient. The normalized far-UV data, representative of secondary structure, were compared to determine if there were statistically significant differences between samples. The two normalization methods agreed in every case, increasing confidence in the results.
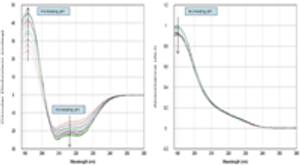
This study describes a new automated technique for measuring protein conformation by CD spectroscopy and how changes can be followed in different pH environments. The study confirms the robustness of the technique and superb sample-to-sample reproducibility.
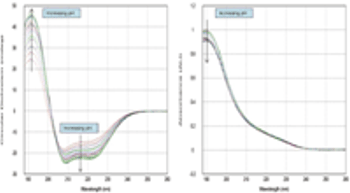
This study describes a new automated technique for measuring protein conformation by CD spectroscopy and how changes can be followed in different pH environments.

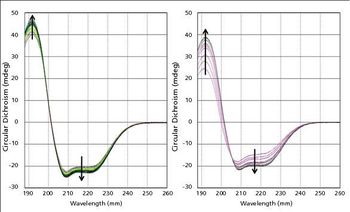
A prerequisite for a successful biotherapeutic formulation is one where the protein is stable and correctly folded. The new technique of dynamic multi-mode spectroscopy (DMS) was used to study the stability of a monoclonal antibody biotherapeutic formulated in acetate and lactate buffers. The samples were measured several times over a period of weeks and it became apparent that the antibody behaved differently as it aged in the two formulations, with the lactate formulation imparting greater robustness than the acetate.