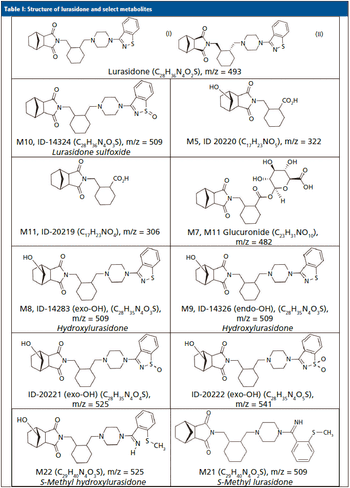
For lurasidone treatment adherence testing, an untargeted high-resolution mass spectrometry method was employed, using known positive human urine samples to identify the lurasidone metabolites and their relative abundance in urine.

For lurasidone treatment adherence testing, an untargeted high-resolution mass spectrometry method was employed, using known positive human urine samples to identify the lurasidone metabolites and their relative abundance in urine.
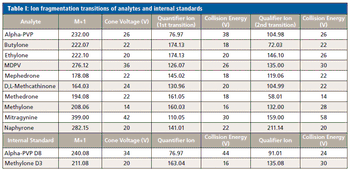
A novel “dilute-and-shoot” LC–MS/MS method is described for the analysis of “bath salts” sold as “legal” highs, including mitragynine and nine synthetic cathinones, in urine.
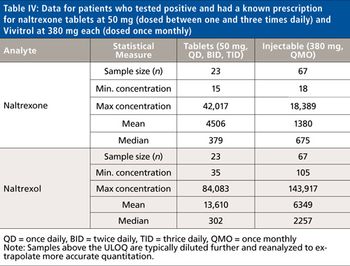
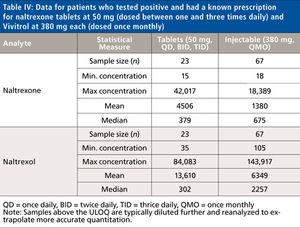
Naltrexone (Depade, Re Via, Trexan) is a potent narcotic antagonist structurally similar to oxymorphone and naloxone. It blocks the subjective effects of heroin and other opiates and is primarily used in the management of opioid dependence and alcohol dependence. While conjugated 6β-naltrexol is the major urinary metabolite in man, conjugated naltrexone and free 6β-naltrexol are also major urinary species. To assist with monitoring substance abuse patients who are prescribed naltrexone, a rapid, selective, and sensitive LC–MS-MS method was developed to analyze for both naltrexone and 6β-naltrexol post-enzymatic hydrolysis. The validation of this method and some representative patient data are discussed in this report.
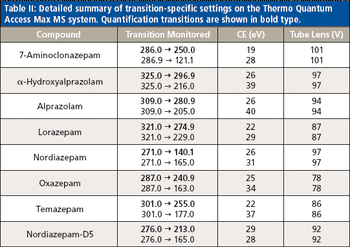
A new method was developed and validated using automated on-line solid-phase extraction (SPE) with tandem mass spectrometry (MS). Urine samples were enzyme-hydrolyzed and diluted before detection. The validated method was applied to positive authentic urine samples to evaluate concordance with high performance liquid chromatography (HPLC)–MS-MS results.
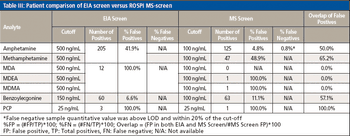
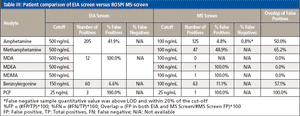
Enzyme immunoassay (EIA) is a conventional drug screening technique, but it can be limited by cross-reactivity that can lead to high false positive rates.
Published: March 1st 2015 | Updated:
Published: May 1st 2015 | Updated:
Published: March 1st 2016 | Updated:
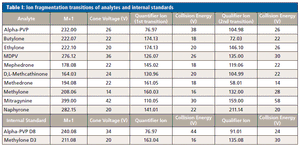
Published: March 1st 2018 | Updated:
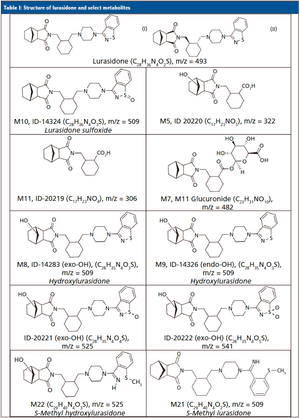
Published: May 1st 2019 | Updated: