Article
Spectroscopy
Spectroscopy
Choosing the Right Atomic Spectroscopic Technique for Measuring Elemental Impurities in Pharmaceuticals: A J-Value Perspective
Author(s):
How to select the optimum atomic spectroscopic technique for orally delivered drugs, based on the J-values described in the validation protocols outlined in USP Chapter
Recent regulations on heavy metal testing have required the pharmaceutical industry to monitor a suite of elemental impurities in pharmaceutical raw materials, drug products, and dietary supplements. These directives are described in the new United States Pharmacopeia (USP) Chapters <232>, <2232>, and <233>, together with The International Conference for Harmonization (ICH) Q3D, Step 4 guideline, which suggests the use of plasma spectrochemistry to measure 24 elemental permitted daily exposure (PDE) levels in the various drug delivery categories. This month's column, adapted from a chapter in Robert Thomas's new book, Measuring Elemental Impurities in Pharmaceuticals: A Practical Guide, offers guidance on selecting the optimum atomic spectroscopic technique for orally delivered drugs, based on the J-values described in the validation protocols outlined in USP Chapter <233>.
Comparing an instrumental technique based on its performance specifications with simple standards is important, but it bears little relevance to how that instrument is going to be used in a real-world situation. For example, instrument detection limits (IDL) are important to know, but how are they impacted by the matrix and sample preparation procedure? What are the real-world method detection limits (MDL), and is the technique also capable of quantifying the maximum concentration values expected for this analysis in the same sample run? And what kind of precision and accuracy can be expected if working close to the limit of quantitation (LOQ) for the overall methodology being used? Additionally, on the sample throughput side, how many samples are expected and at what frequency will they be coming into the laboratory? How much time can be spent on sample preparation and how quickly must they be analyzed? Sometimes the level of interferences from the matrix will have a major impact on the selection process or even the amount and volume of sample available for analysis (1,2).
Understanding the demands of an application is therefore of critical importance when a technique is being evaluated, and particularly if there is a minimum amount of expertise or experience in house on how best to use it to solve a particular application problem. This could be the likely scenario in a pharmaceutical or dietary supplement production laboratory that is being asked to check the elemental impurities of incoming raw materials used in the manufacturing process. They will now have to follow the new United States Pharmacopeia (USP) Chapters <232> (or Chapter <2232> for dietary supplements) and <233> (3), which recommend the use of a plasma-based spectroscopic technique to carry out the analysis-or any other atomic spectroscopy technique as long as the validation protocols are met. Note: The International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals (ICH) has it's own directives, which are described in its Q3D Step 4 guidelines (4).
The expertise of the operator should never be underestimated, because if inductively coupled plasma–mass spectrometry (ICP-MS) is being seriously considered, it generally requires an analyst with a higher skill level to develop rugged methodology free of interferences that can eventually be put in the hands of an inexperienced user to operate on a routine basis. Again, this is a real concern if the technique is being used by novice users who have limited expertise in running analytical instrumentation, which may be the case in the pharmaceutical or nutraceutical industries. So, let's take a closer look at the new USP chapters to understand what atomic spectroscopy techniques might best meet the demands of this application.
Suitability of Technique
To get a better understanding of the suitability of the technique being used and whether its detection capability is appropriate for pharmaceutical materials, it's important to know the permitted daily exposure (PDE) limit for each target element. In particular, it's important to understand what the USP calls the J-value, as described in Chapter <233>, which is defined as the PDE concentration of the element of interest, appropriately diluted to the working range of the instrument, after the sample preparation procedure to get the sample into solution is completed. Let's use Pb as an example. The PDE limit for Pb in an oral medication defined in Chapter <232> is 5 µg/day.
Based on a suggested dosage of 10 g of the drug product/day, that's equivalent to 0.5 µg/g Pb. If 1.0 g of sample is digested or dissolved and made up to 500 mL, that's a 500-fold dilution, which is equivalent to 1.0 µg/L. So, the J-value for Pb in this example is equal to 1.0 µg/L.
The method then suggests using a calibration made up of two standards: standard 1 = 1.5J, standard 2 = 0.5J. So for Pb, that's equivalent to 1.5 µg/L for standard 1 and 0.5 µg/L for standard 2.
The suitability of a technique is then determined by measuring the calibration drift and comparing results for standard 1 before and after the analysis of all the sample solutions under test. This calibration drift should be <20% for each target element. However, once the suitability of the technique has been determined, further validation protocols described in detail in Chapter <233>, must be carried out to show compliance to the regulatory agency if required.
It should also be pointed out that no specific instrumental parameters are suggested in Chapter <233>, but only to analyze according to the manufacturer's suggested conditions and to calculate and report results based on the original sample size. However, it does say that appropriate measures must be taken to correct for interferences, such as matrix-induced wavelength overlaps in inductively coupled plasma–optical emission spectrometry (ICP-OES) and argon-based polyatomic interference with ICP-MS.
Let's examine this approach by taking an example of measuring 24 elemental impurities in an oral drug according to Chapter <232> and calculating the J-values for each elemental impurity and comparing them with the limits of quantitation (LOQ) for each atomic spectroscopy technique (such as atomic absorption, axial-ICP-OES, and ICP-MS) to give us an assessment of their suitability. For this analytical scenario, we'll take the LOQ for the technique as 10x the IDL. These LOQs were calculated by taking the average of published IDLs from three instrument vendors' application material and multiplying them by 10 to get an approximation of LOQ. In practice, a method limit of quantitation is typically determined by processing the matrix blank through the entire sample preparation procedure and taking 10 replicate measurements. The method LOQ, sometimes referred to as the method detection limit, is then calculated as 3–7x standard deviations of these 10 measurements, depending on the percent confidence level required.
To make this comparison valid, the sample weight was adjusted for each technique, based on the detection limit and analytical working range. For atomic absorption and ICP-OES, we used a sample dilution of 2 g/100 mL, whereas for ICP-MS we used 0.2 g/100 mL. Atomic absorption and ICP-OES could definitely use larger sample weights, but for high-throughput routine analysis, we are probably at the optimum dilution for ICP-MS. (Note: For this assessment, it was felt that axial ICP-OES was the better choice over the radial configuration, because of its superior detection capability.)
Tables I–III show the comparison of atomic absorption (flame and furnace), axial ICP-OES, and ICP-MS, respectively, for 24 elemental impurities in an oral drug with a maximum dosage of 10 g/day, according to Chapter <232>. The important data to consider is in the final column, labeled "Factor Improvement," which is the J-value, divided by the LOQ. Generally speaking, the higher this number the more suitable the technique.
The "Class" column in these tables indicates the level of toxicity of the elements, which have been determined based on chronic exposure data and likelihood of occurrence in the drug product. (It is generally recognized that Class 1 and 2A elements are the most important to monitor.) Also it should be noted that the arsenic and mercury PDEs are based on the inorganic forms of the element.
Relationship Between LOQ and J-Value
It should be emphasized again that LOQ in these examples is just a guideline as to the real-world detection capability of the technique for this method. However, it does offer a very good approximation as to whether the technique is suitable based on the factor improvement number compared to the J-values for each elemental impurity. Clearly, if this improvement number is close to or less than 1, as it is with the majority of elements by flame atomic absorption (FAA), the technique is just not going to be suitable, particularly for the "big four" heavy metals, which are the most critical. On the other hand, electrothermal atomization (ETA) would be suitable for the majority of the impurities (including the heavy metals), except for a few of the catalyst-based elements, which are not ideally suited to the technique. However, the ETA technique is very time-consuming and labor intensive, so it probably wouldn't be a practical solution in a high-throughput pharmaceutical production laboratory. The comparison between FAA and ETA is exemplified in Table I.
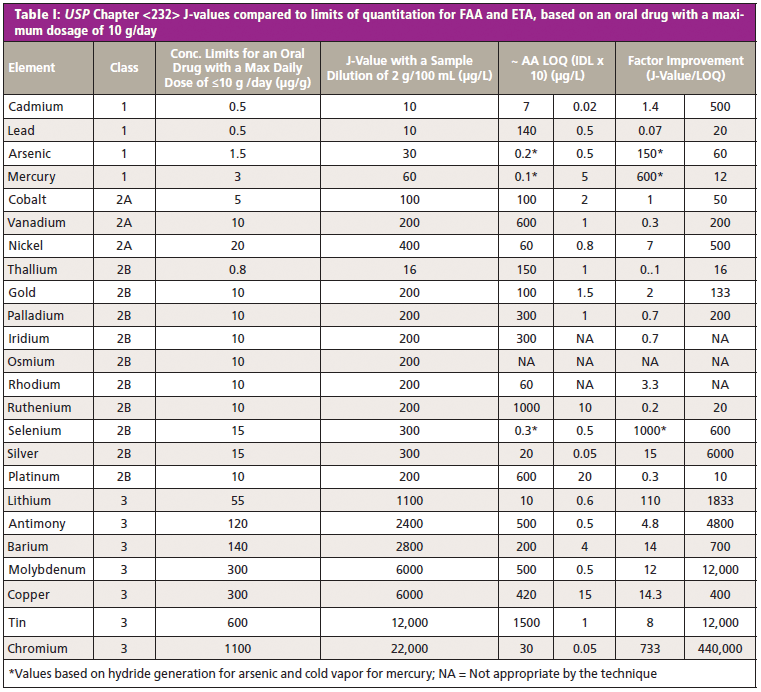
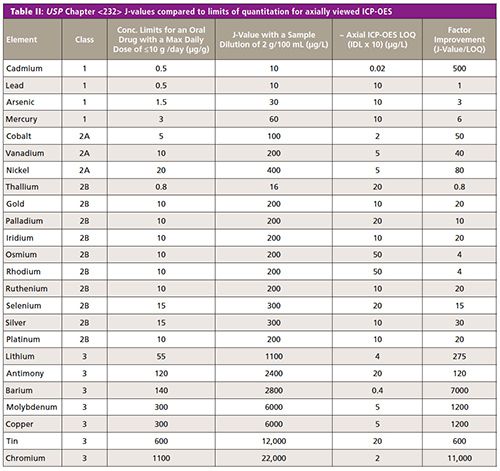
Table II shows that axial ICP-OES offers some possibilities for monitoring oral drugs because the vast majority of the improvement factors are higher than 1. These numbers could be further improved, especially for the heavy metals, by using a much higher sample weight in the sample preparation procedure without compromising the method. (Note: Because most commercial ICP-OES instrumentation offer both axial and radial capability [dual-view], it was felt that the axial performance was most appropriate for this comparison.)
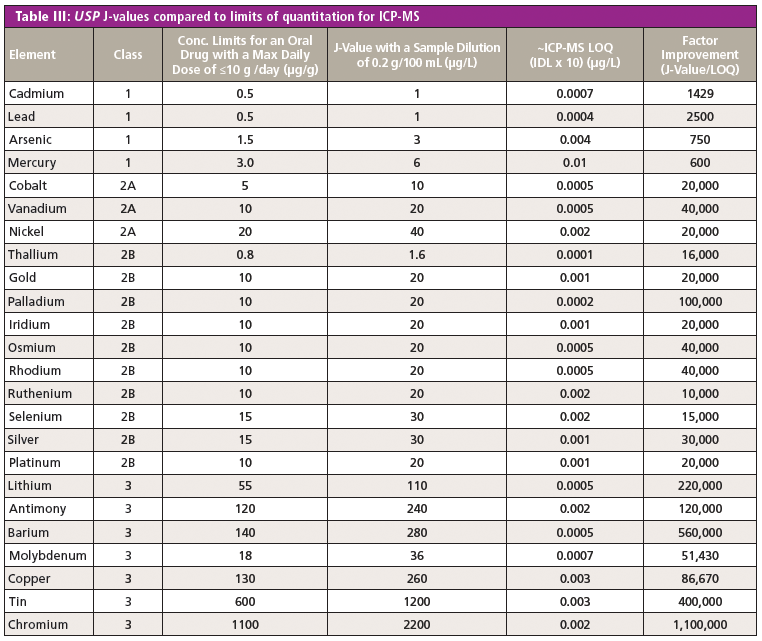
However, it can be seen in Table III that ICP-MS shows significant improvement factors for all impurities, which are not offered by any other technique. Even for the four heavy metals, there appears to be ample improvement to monitor them with good accuracy and precision. The added benefit of using ICP-MS is that it would also be suitable for the other methods of pharmaceutical delivery, such as parenteral or inhalation, where the PDE levels are typically one or two orders of magnitude lower. Additionally, if arsenic or mercury levels were found to be higher than the PDE levels, it would be relatively straightforward to couple ICP-MS with high performance liquid chromatography (HPLC) to monitor the speciated forms of these elements if required.
Final Thoughts
It is important to understand that there are many factors to consider when selecting a trace element technique that is most suited to the demands of your application. Sometimes, one technique stands out as being the clear choice, whereas other times it is not quite so obvious. And, as is true with many applications, more than one technique is often suitable. However, the current methodology described in the new USP Chapters <232> and <233> presents unique challenges, not only from a perspective of performance capability, but also because of the validation protocols that have to be met to show suitability of the technique to the analytical procedure being used. From a practical standpoint, there is no question that to meet all the PDE limits in all pharmaceutical delivery methods, particularly for parenteral and inhalation drugs where the PDEs are significantly lower, ICP-MS is probably the most appropriate technique. However, for oral delivery products, especially liquid medications or those that can be easily brought into solution with a suitable aqueous or organic solvent, axial ICP-OES could offer a more cost-effective approach. In addition, ICP-OES can use larger sample weights and lower dilutions, which will improve its detection capability. In addition, if the sample workload requirements are not so demanding, ETA could provide a solution for monitoring a small number of samples for the critical Class 1 and 2A elements. However, I think it's fair to say that ICP-MS has shown it has the detection limits and throughput capability to be the optimum technique of choice for carrying out the measurement of elemental impurities in a wide and diverse range of pharmaceutical materials.
Note: Elemental contaminants in dietary supplements were not evaluated for this article, but a full explanations of the J-value calculations compared to the limit of quantitation for the four elemental PDEs specified in Chapter <2232> are given in a chapter of the new book cited in the abstract (5).
References
(1) R.J. Thomas, Today's Chemist at Work, October, 1999.
(2) R.J. Thomas, International Labmate (2016), https://www.labmate-online.com/article/mass-spectrometry-and-spectroscopy/41/scientific-solutions/money-to-burn-do-you-know-what-is-costs-to-run-your-atomic-spectroscopy-instrumentation/2030.
(3) Elemental Impurities in Pharmaceuticals: Updates: USP Website: http://www.usp.org/chemical-medicines/elemental-impurities-updates.
(4) International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use, http://www.ich.org/products/guidelines/quality/article/quality-guidelines.html.
(5) R.J. Thomas, Measuring Elemental Impurities in Pharmaceuticals: A Practical Guide (CRC Press, Boca Raton, Florida, 2018), ISBN 13:978-1-138-19796-1.
Robert Thomas

Robert Thomas is principal of Scientific Solutions, a consulting company that serves the application and writing needs of the trace element user community. He has worked in the field of atomic and mass spectroscopy for more than 40 years and has written over 80 technical publications including a 15-part tutorial series on ICP-MS. He recently completed his fourth textbook entitled Measuring Elemental Impurities in Pharmaceuticals: A Practical Guide. He has an advanced degree in analytical chemistry from the University of Wales, UK, and is also a Fellow of the Royal Society of Chemistry (FRSC) and a Chartered Chemist (CChem). He has served on the ACS Committee on Analytical Reagents for the past 17 years.

Newsletter
Get essential updates on the latest spectroscopy technologies, regulatory standards, and best practices—subscribe today to Spectroscopy.