Protein secondary structure during thermal unfolding and aggregation is readily acquired using IR spectroscopy and a temperature-controlled mid-IR transmission accessory. Myoglobin was used as a model system to illustrate the method.

Protein secondary structure during thermal unfolding and aggregation is readily acquired using IR spectroscopy and a temperature-controlled mid-IR transmission accessory. Myoglobin was used as a model system to illustrate the method.

Protein secondary structure during thermal unfolding and aggregation is readily acquired using IR spectroscopy and a temperature-controlled Mid-IR transmission accessory.
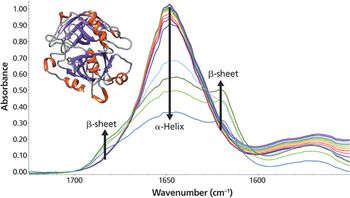
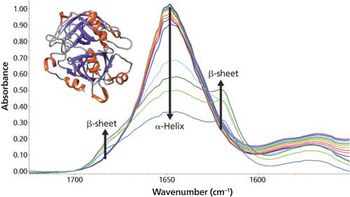
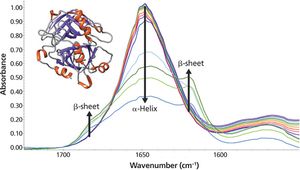
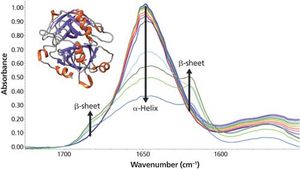
As the temperature increases, significant changes in the myoglobin secondary structure are observed. Between 25 °C and 70 °C, the 1649 cm-1 peak height decreases and linewidth increases. These changes in the amide I band reflect increased protein structural disorder. A temperature increase from 70 °C to 75 °C causes a drop in the 1649 cm-1 band intensity. Simultaneously, two peaks grow in at 1683 and 1637 cm-1. These changes indicate conversion of random coil/α-helix to intermolecular β-sheet. Further conversion to β-sheet is observed as the temperature rises to 90 °C. The final β-sheet structure is an insoluble aggregate. This aggregation process is not reversed when the temperature is driven back to 25 °C.
Published: February 1st 2016 | Updated:

Published: March 16th 2016 | Updated:
Published: April 1st 2016 | Updated: