
How to create trouble-free sample preparation workflow for elemental analysis.

How to create trouble-free sample preparation workflow for elemental analysis.

The determination of metals in hygienic face masks is important for global heath at this time. Following the guidelines of ISO 18562-4, an ICP-OES method is described including sample preparation, analysis, and validation of the methodology.

John W. Olesik of The Ohio State University discusses his collaborative ICP–OES and ICP–MS research and shares insights gleaned over a career of teaching and research.

Although ICP-OES and ICP-MS are often considered mature techniques, probing the mechanisms involved can lead to more accurate results, and in some cases, cast doubt on accepted explanations. John Olesik shares some surprises he has encountered, in this first part of a two-part interview series.

Inductively coupled plasma–atomic emission spectroscopy (ICP-AES) relies on the use of a peristaltic pump for sample introduction. Here, two conventional peristaltic pumps are compared with a new pump based on the “easy click” principle for the analytical figures of merit.

There is no doubt that funding for basic science in analytical atomic spectroscopy has waned over the years, and, in reality, barely exists today.

In celebration of Spectroscopy’s 35th Anniversary, leading experts discuss important issues and challenges in analytical spectroscopy.
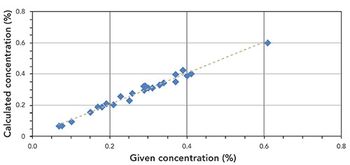
New excitation developments and advanced detector technology enable the use of EDXRF for multielement analysis of plant material and fertilizers, with improved detection limits and reduced measurement time. These features are combined with easy sample preparation and low cost of investment.

EDXRF offers potential advantages over ICP-OES for elemental analysis in agriculture. Karen Daly and Anna Fenelon of the Agriculture and Food Development Authority of Ireland spoke to us about their work investigating agricultural applications of this technique.
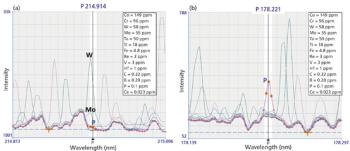
Master the art of selecting analytical wavelengths for ICP-OES with these essential steps and enhance your results.
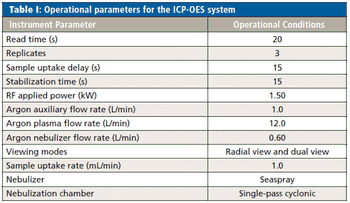
This method demonstrates that ICP-OES is a suitable alternative to ICP-MS for the determination of rare earth elements in geological and agricultural samples
The nutritional value of food depends on many components, including vitamins and minerals. While both of these occur naturally, they are also commonly added during processing to increase the nutritional content. Naturally occurring nutrients enter plants (and ultimately animals who consume plants) from the soils in which they grow, so it is equally important to monitor the nutrient content of both soil and final food products. Since the number of elemental nutrients is limited and they are present at relatively high concentrations, ICP-OES is an ideal technique for their measurement in soil and food. This work will focus on the elemental nutrient analysis of soils and two categories of food products: milk and fruit juice, whose nutritional content is particularly important as they are commonly consumed by young children.

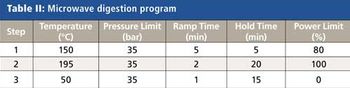
October’s AP column highlights a team of geochemists at the University of Houston who have been developing methods to streamline multi-element analysis for a more complete fingerprinting of oils by using one sample preparation method utilizing a single reaction chamber microwave digestion system and then analyzing these solutions for major, and minor elements by ICP-OES and low abundance trace elements by triple quadrupole (QQQ) ICP-MS. Results to date using this approach have shown that complete elemental recovery and removal of organic matrices can be achieved safely and that up to 57 elements can be determined in oils with good accuracy and precision. Removal of organic matrices during digestion not only helps to limit the formation of polyatomic spectral interferences, but improves instrument stability and reduces carbon build in the sample introduction and interface regions, which have traditionally plagued “dilute and shoot” methods.
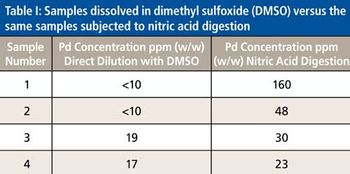
The use of atomic spectroscopy techniques and sample preparation procedures is something that is not as routine in the pharmaceutical industry as are chromatography-based techniques and sample preparation procedures. With new requirements being implemented regarding elemental impurities by the United States Pharmacopoeia (USP) and International Conference on Harmonization (ICH), analysts in the pharmaceutical industry are, in many cases, working to determine how best to analyze their samples. Sample preparation techniques that can be used for pharmaceutical samples are the same ones that have been used by other industries for many years. This paper will provide a brief overview of potential techniques.
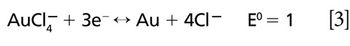
Efficient and accurate measurement of mercury concentration is a challenge. A direct sample preparation method for reliable ICP-OES mercury measurement would be invaluable to chemical manufacturers, testing laboratories, and other industries. Historically, ICP-OES Hg measurements have been plagued by poor Hg detection limits, severe carryover effects, and sample instability. In this study, we present a method of sample preparation for ICP-OES mercury analysis in various reagent chemical compounds. This sample preparation method is straightforward and direct, allowing mercury analysis in a variety of reagent chemicals without digestion.
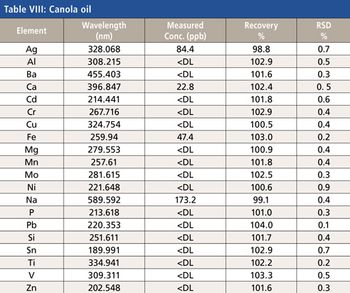
This paper describes the analysis of edible oils radial view ICP-OES. Information is provided regarding the most suitable wavelengths, background correction, and integration times. Results of a detection limit study are presented. The accuracy of the analytical method is validated using soybean, olive, and corn oil matrices.
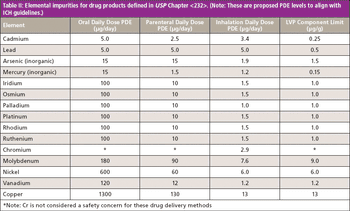
This installment evaluates the application requirements for the determination of 15 elemental impurities in pharmaceutical materials as described in the new United States Pharmacopeia (USP) Chapters and and offers suggestions about which atomic spectroscopic technique might be the most suitable to use.
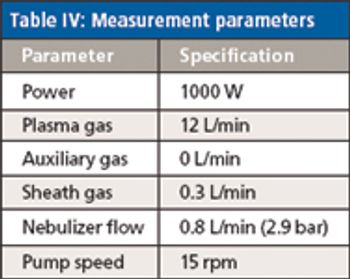
Rare earth elements (REEs) have become indispensable in many electronic, optical, magnetic, and catalytic applications because of their specific properties such as magnetism and phosphorescence, as well as their ability to both donate and accept electrons.

A new sodium peroxide fusion method is described, as well as the conditions for inductively coupled plasma–optical emission spectrometry, and a list of the accuracy and precision measurements for all prepared samples.

A critical review of the main developments in instrument technology, calibration, and sample preparation that have made it possible to determine low sulfur concentrations in fuels followed by a discussion of strategies to minimize spectral interferences related to sulfur determination by ICP-MS, such as collision–reaction cells, high-resolution mass analyzers, and the interference standard method.

The USP proposes the use of analytical techniques capable of measuring impurities at the specified limits with optimal selectivity, sensitivity, simplicity, and robustness.
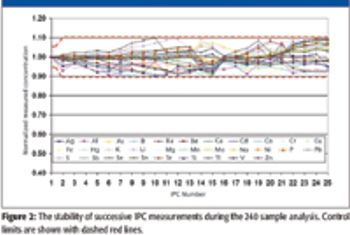
ICP-AES, specified by the U.S. EPA Method 200.7, is capable of fulfilling requirements for analyzing a total of 32 metals and trace elements in supplied water, natural water, and wastewater. Here, the authors look at this method and its application to water analysis.

Analyis of lead paint in toys using ICP-OES
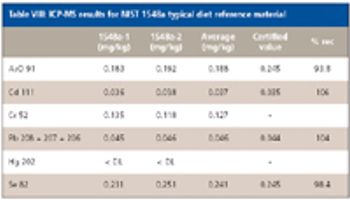
The determination of inorganic elements in food substances is critical for assessing nutritional composition and identifying food contamination sources. The inorganic elements of interest can be divided into two classes: nutritional and toxic. It is important to determine the levels of both sets of elements accurately to assess both the nutritional and the harmful impacts of food substances. Nutritional elements such as Mg, P, and Fe are present at high levels (milligrams per kilogram), while toxic elements such as Pb, Hg, and Cd should be present only at trace levels (nanograms or micrograms per kilogram).
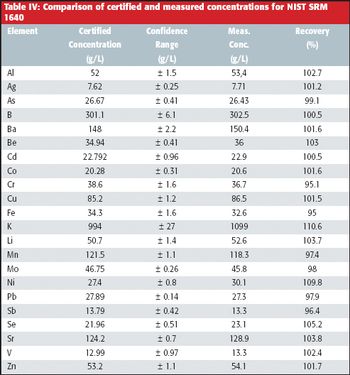
The multielement analysis of water is one of the major applications for inductively coupled plasma-optical emission spectroscopy (ICP-OES). This report describes the analysis of metals and trace elements in drinking water in terms of sensitivity, precision, and accuracy. Instrument parameters and line selection are described. Excellent recoveries were found for the standard reference material (SRM) NIST SRM 1640.