
The authors present the results of a study in which FT-IR-ATR and FT-Raman spectrosopies were used to probe the effects of pasteurization and spray drying on the secondary structure of soy protein isolate.

The authors present the results of a study in which FT-IR-ATR and FT-Raman spectrosopies were used to probe the effects of pasteurization and spray drying on the secondary structure of soy protein isolate.

January 2007. This review article summarizes biological applications that utilize surface plasmon resonance, localized surface plasmon resonance, and surface-enhanced Raman spectroscopy.

Here the authors describe a new method for the identification of key volatile organic compound markers using mass spectrometry combined with gas chromatography.
In this article, the role of a triple-quadrupole mass spectrometer in performing in vitro studies of compound metabolic stability and identification of Phase I and II metabolites is demonstrated.

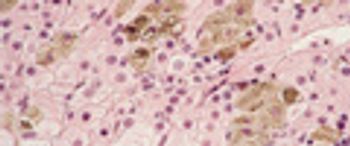
IR imaging provides a new tool for disease detection, revealing compositional and structural information in tissue previously not available with contrast staining.

The concentration dependent influence of Na+ and K+ions on mass spectra of peptides is shown with human gastrin as a model peptide. With electrospray ionization the doubly charged protonated molecule ion [M+2H]2+ is normally the preferred ionization product. However, trace amounts of alkali metal ions already form clusters (adducts) with the peptide molecule, such as [M+H+Na]2+, which become dominating at higher concentrations. With Na+/K+ concentrations below 0.1 mg/kg (ppm) only a few clusters appear, which allow the correct doubly charged molecule ion to be assigned for a subsequent MS–MS experiment. With concentrations of 10 ppm and higher the alkali clusters become the most abundant peaks in the spectrum, and the absolute sensitivity is decreased by a factor of 5–10. Experiments were performed with water and water–methanol mixtures with a known Na+/K+ +content.
Many important biological signals are triggered by the binding of a peptide hormone to its cognate receptor at the cell surface. Using stopped-flow fluorescence spectroscopy, the authors have been able to observe, in real time, ligand binding to epidermal growth factor receptors expressed at the surface of intact cells. This method allows for the measurement of kinetic association and dissociation rates with high data density in a native cellular environment, providing insights into the signal-initiation process in this system that have not been revealed through the determination of ligand-binding constants obtained by more traditional methods.

The element selenium plays three distinct roles in biological processes, functioning in turn as a toxicant, a chemopreventive agent, and a heavy metal antagonist. This article discusses current research associated with each role, and how ICP-MS can be employed to better understand and utilize selenium's properties.