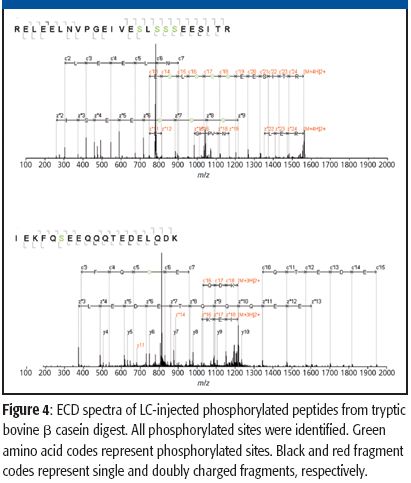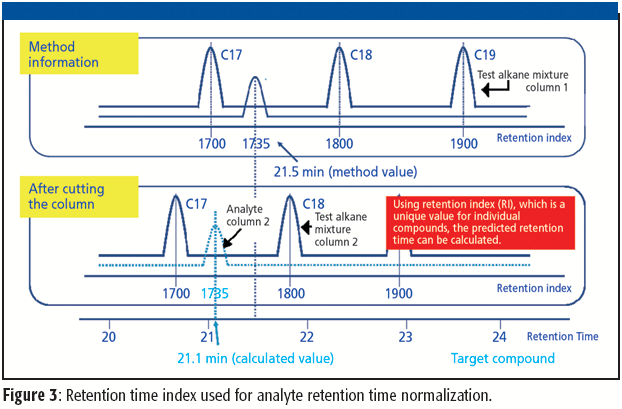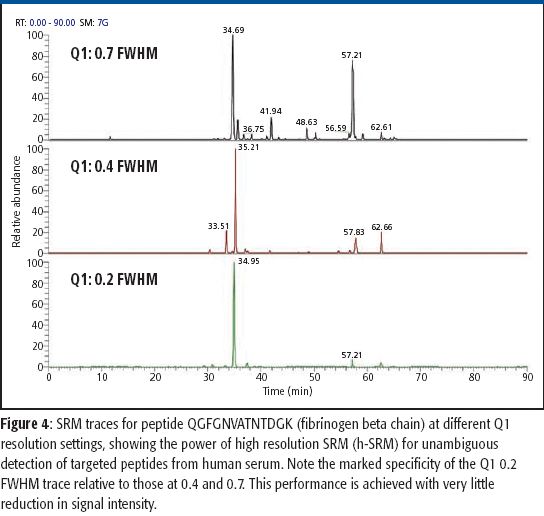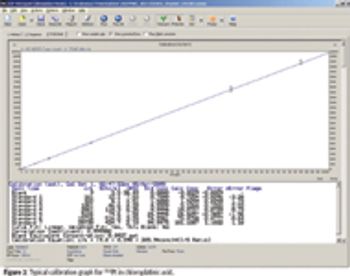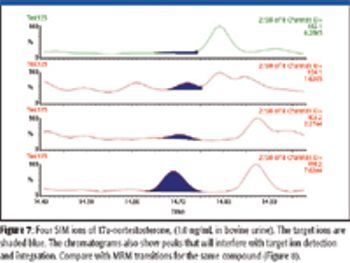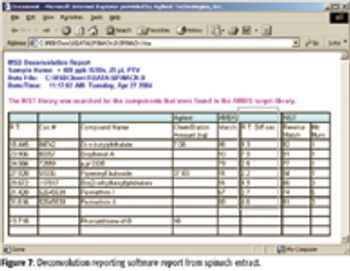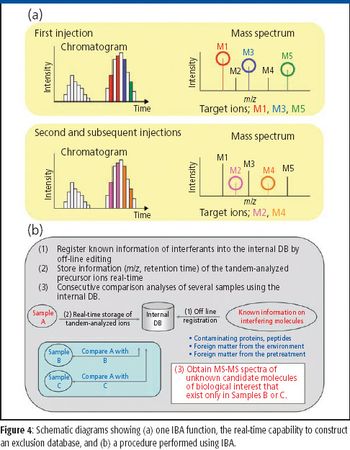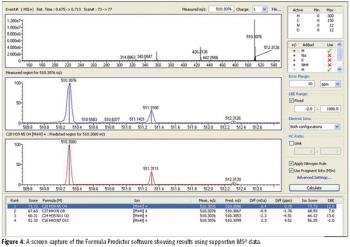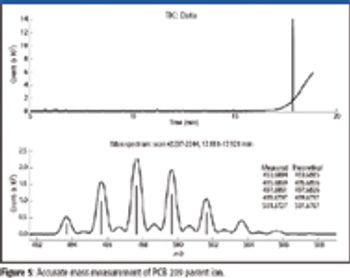
The ability to perform accurate mass measurements in mass spectrometry (MS) for elemental composition determination (ECD, also known as formula identification) provides a powerful tool for assisting in the identification of unknown compounds. Recent advances in data processing methods have demonstrated the ability to obtain mass accuracy in the 5–10 ppm range on routine single- and tandem-quadrupole systems (1,2), sufficient to assist in the formula identification. However, even on more expensive high-resolution systems such as quadrupole time-of-flight (qTOF) or Fourier transform (FT)–MS instruments that are capable of routinely measuring mass accuracy in the 1–3 ppm range, the formula identification is not unique, particularly for higher molecular weight compounds. By calibrating instruments to obtain high spectral accuracy as well as mass accuracy, the ability to unambiguously identify the formula is improved substantially, particularly on low-resolution systems.