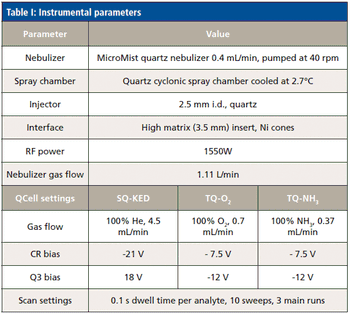
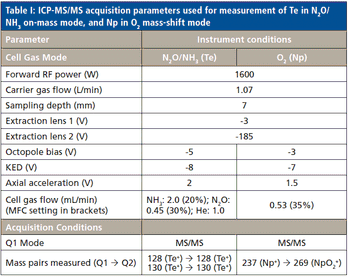
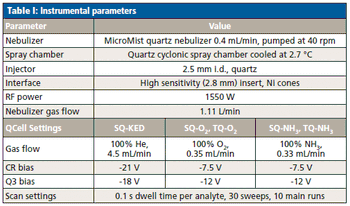
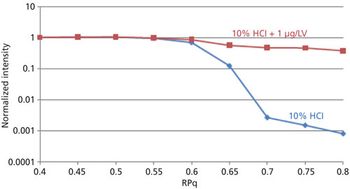
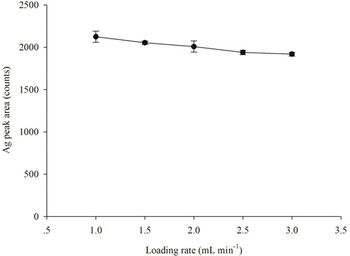
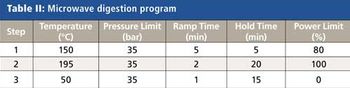
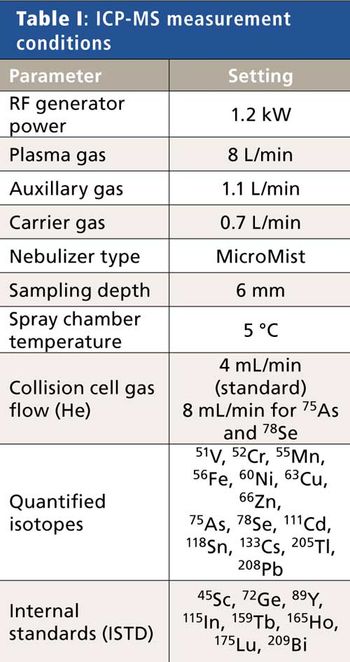
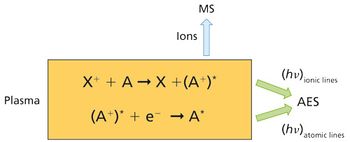
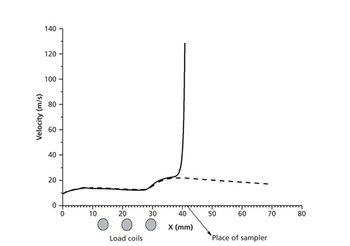
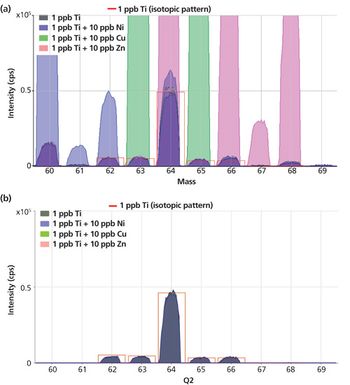
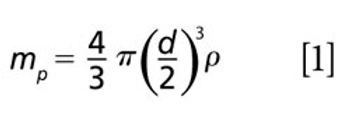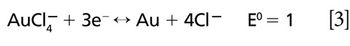The detection, quantitation, and characterization of nanoparticles using inductively coupled plasma–mass spectrometry (ICP-MS), and in particular using single-particle ICP-MS (SP-ICP-MS), has developed significantly in recent years. However, the difficulties involved in this type of analysis vary, depending on the composition of the nanoparticles. Martín Resano of the University of Zaragoza, together with colleagues from Ghent University, has recently developed a method for characterizing nanoparticles made from silicon dioxide (Si02), which are much more challenging to detect than those made from silver or gold. He recently spoke to us about this work.