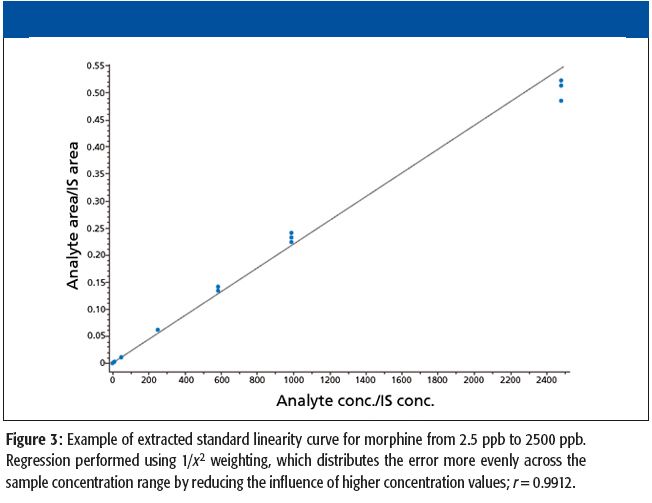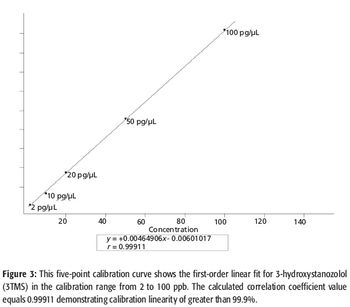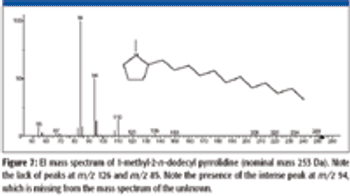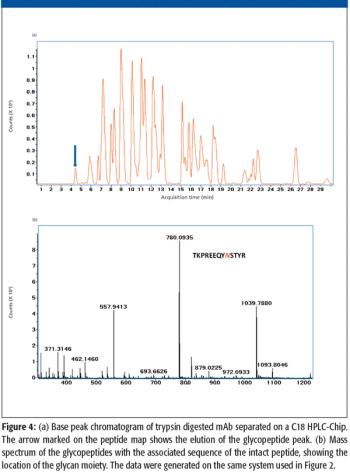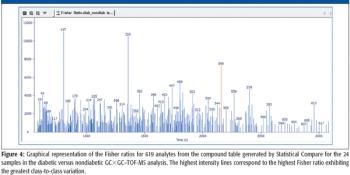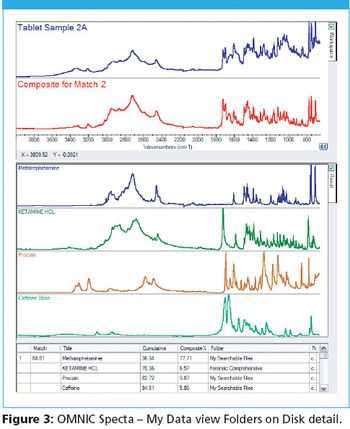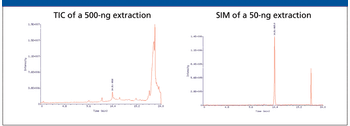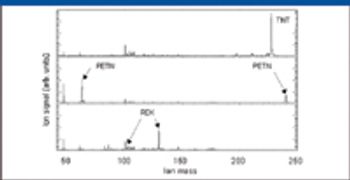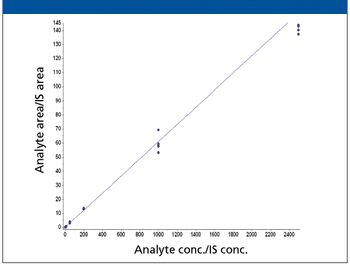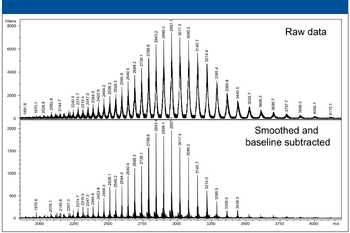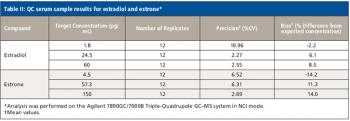
A number of clinical situations now call for high-sensitivity measurement of estrogens, including monitoring during female hormone replacement therapy, antiestrogen treatment, and estrogen deficiency in men. Traditional immunoassay methods and liquid chromatography–tandem mass spectrometry (LC–MS-MS) do not provide the sensitivity and selectivity required for these applications. In contrast, a gas chromatography–negative chemical ionization–tandem mass spectrometry (GC–NCI-MS-MS) platform can provide detection limits below 1 pg/mL when used in conjunction with the appropriate derivatization protocol, with very short cycle times.