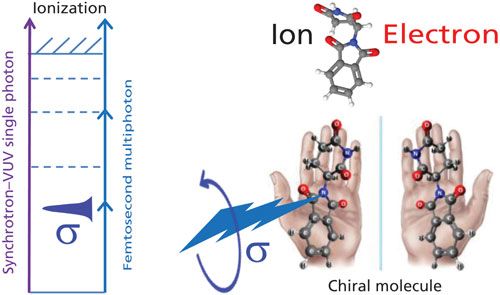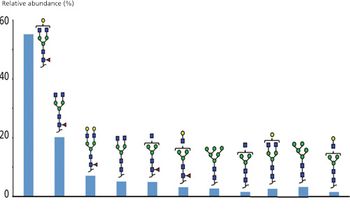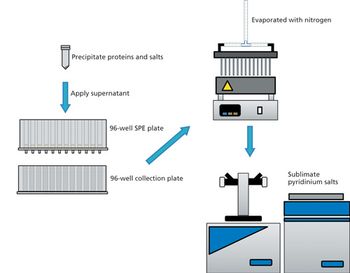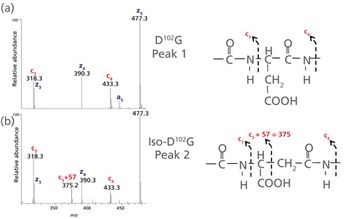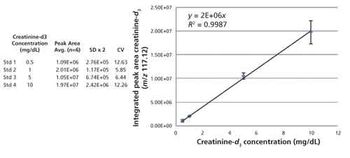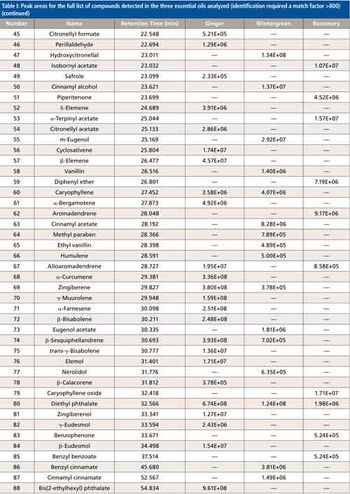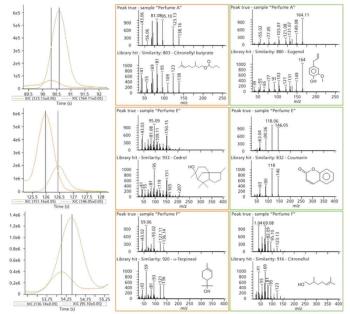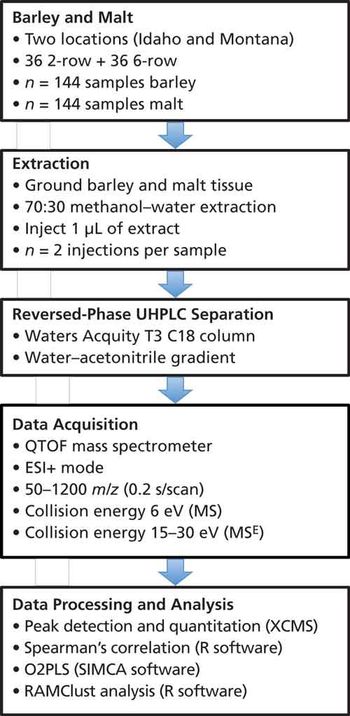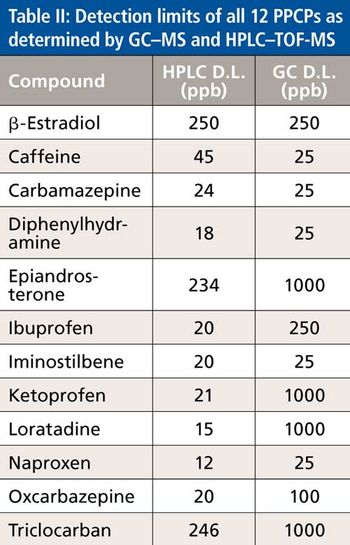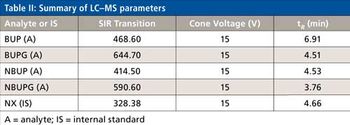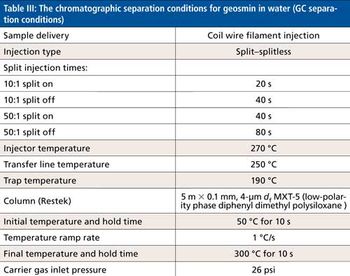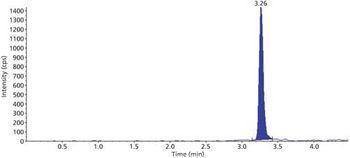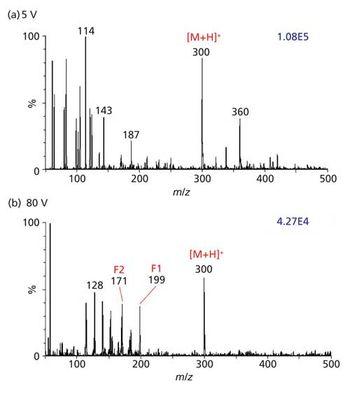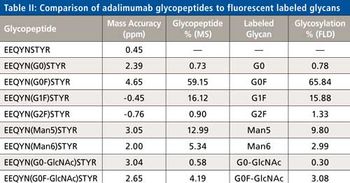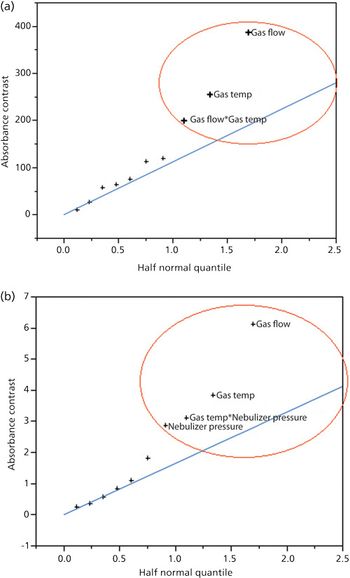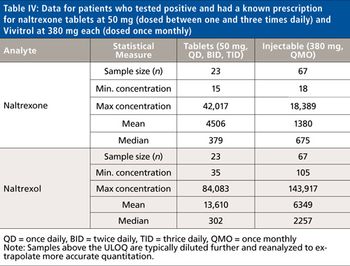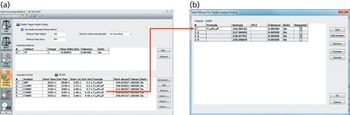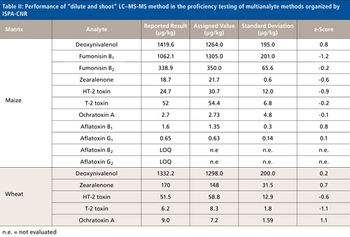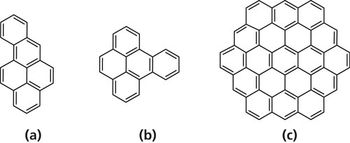
Quantum chemistry is capable of calculating a wide range of electronic and thermodynamic properties of interest to a chemist or physicist. Calculations can be used both to predict the results of future experiments and to aid in the interpretation of existing results. This paper will demonstrate some examples where quantum chemistry can aid in the development of mass spectrometric methods. Gas-phase electron affinities (EAs) have been difficult to determine experimentally, so the literature values are often not reliable. Computational methods using quantum chemistry have allowed the compilation of a self-consistent database for the EAs of polynuclear aromatic compounds. Likewise, proton affinities (PAs) and ionization potentials (IPs) have been calculated and compared favorably with experimental results for these molecules.