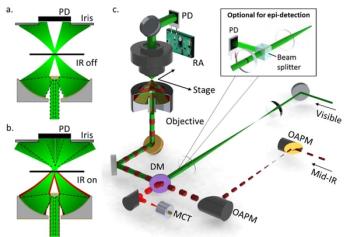
Significant progress is being made to harness the power of spectroscopy technique for medical research. An ongoing challenge, and area of development, in this effort, is to “see” more and more detail about biological activity, even within individual cells. Ji-Xin Cheng, a professor of biomedical engineering at Boston University, is advancing such work, by developing techniques like midinfrared photothermal (MIP) imaging and Raman spectromicroscopy. Cheng is the 2019 winner of the Ellis R. Lippincott Award, which is awarded annually by the Optical Society, the Coblentz Society, and the Society for Applied Spectroscopy, to an individual who has made significant contributions to the field of vibrational spectroscopy. Here, Cheng speaks to us about those techniques.