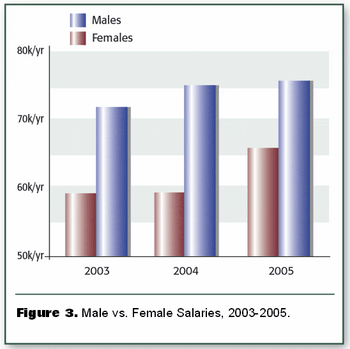
Spectroscopy's annual look at practiitioners' incomes and attitudes about their jobs finds that both have improved modestly in the face of challenging economic and employment conditions.

Spectroscopy's annual look at practiitioners' incomes and attitudes about their jobs finds that both have improved modestly in the face of challenging economic and employment conditions.

The author discusses an instrument called SEARCH (Scan for Extinct Astrobiological Residues and Current Habitats) designed for use on NASA Mars rover missions.

The authors present an MS technique that reliably detects several types of fungal mycotoxins, and compare electrospray ionization and atmospheric chemical ionization techniques.

The authors describe implementation and performance of a miniaturized NIR analyzer and associated chemometric techniques applied to the monitoring of a drying process for active pharmaceutical ingredients (APIs).

This article introduces the application of high-resolution ultrasonic spectroscopy for the analysis of emulsions and suspensions.

This article provides an overview of several recently developed technologies that will improve the utility and performance of mass spectrometers in a number of applications.

The authors describe an ICP detection technology that combines photo-current conversion and a solid-state multichannel detector.

The need to verify cleaning between manufacturing runs presents a special challenge to the analytical chemist. In this article, the principles of ion mobility spectrometry are described, its performance is compared to HPLC for the analysis of cleaning validation samples, and findings are presented from a study to establish the feasibility of using IMS in validating a cleaning verification method.

A majority of current discussion about NIR's applicability to pharmaceutical manufacturing is focused on preparative processes such as granulation, drying, and blending. The author examines different considerations for NIR for the output of the manufacturing line - finished tablet analysis.

This article reviews the development of FDA's PAT Initiative along with CPAC's ANSI/ISA-76.00.02 (2002) (NeSSI) development. The intent is to show how the NeSSI components could contribute to the PAT philosophy and physical requirements.

A paradigm shift is required for chemists and engineers to best utilize chemometrics in their processes. This change demands that one not be too fixated upon ideal textbook thermodynamic models but instead continually check these models using real-time data input and chemometric analysis. The author discusses implementation strategies and the benefits that chemometrics can bring to the process environment.

The authors describe the in-line moisture measurement of a pharmaceutical granulation of lactose, microcrystalline cellulose, and crospovidone in a fluid bed granulator-dryer using top sprayed granulating liquid. A near-infrared (NIR) prediction model was developed for moisture on spectra collected during a calibration run. Subsequent granulations were analyzed for moisture content real-time throughout the granulation and drying process using the NIR process instrument.

What does the accelerating adoption of PAT-based approaches to pharmaceutical manufacturing mean for the makers and users of spectroscopic systems?

NIR-CI adds a completely new dimension to conventional NIR spectroscopy.

Mass spectrometry systems have specific vacuum requirements. New developments in oil-free, or dry, primary vacuum pumps have been introduced recently and are discussed in this article with respect to capacity, throughput, and specific pumping requirements for process gases.

Encoded photometric technology has the ability to address the demands of modern process applications, including those of the PAT initiative.

This article discusses the key components of a typical liquid sample introduction system for inductively coupled plasma spectroscopy, and offers troubleshooting tips for problems commonly encountered by practitioners.

Through symposia, posters, and workshops, representatives from industry and academia addressed a broad range of technology and implementation issues that are beginning to change the landscape of modern spectroscopy.

The authors at how linearity considerations fit into the broader scheme of calibration theory, and discuss methods for testing data for non-linearity.

This paper describes how analysts at an environmental and geochemical laboratory utilize dynamic reaction cell ICP-MS technology to study the transport and deposition of important trace metals from rainwater.

The author provides a brief historical and technological review of different types of telescopes, and how they were developed and improved.

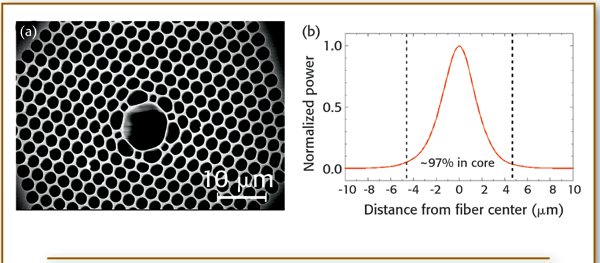
FiberTech Optica Corporate Profile

SCP SCIENCE Corporate Profile

SCP SCIENCE Corporate Profile

SPEX CertiPrep Corporate Profile